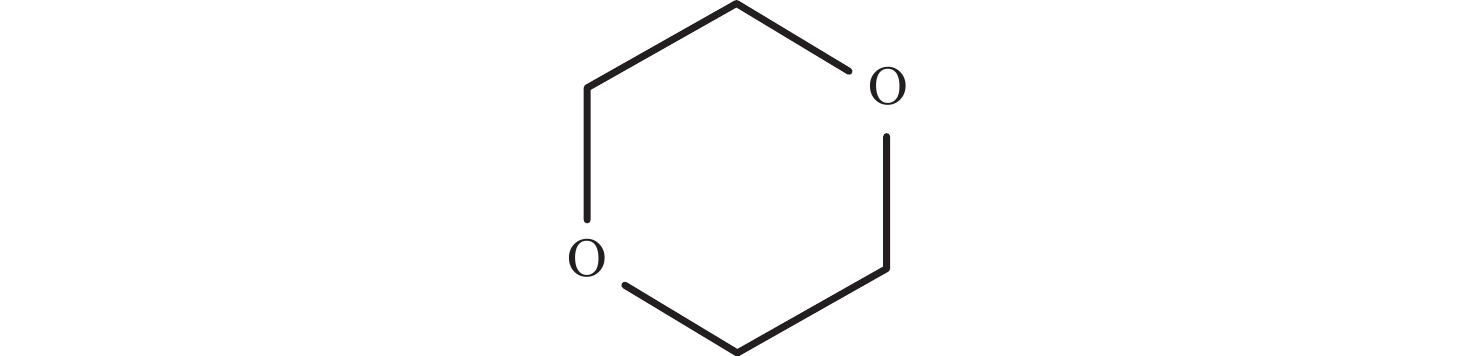
Current status and prospects of research on 1,4-dioxane pollution and treatment technologies in the water environment
-
Abstract: 1,4-dioxane pollution is characterized by its early identification, widespread sources and extensive distribution. The pollutant is highly mobile and persistent in the water environment and is classified as a B2 (probable) human carcinogen. After reviewing recent researches on the pollution status, transport and transformation characteristics of 1,4-dioxane in the water environment, as well as the environmental pollution remediation and treatment technologies, and the status of environmental regulation, this paper addresses that the distribution of 1,4-dioxane in water bodies is significantly correlated with chlorinated hydrocarbon pollutants such as 1,1,1-trichloroethane (1,1,1-TCA) and trichloroethylene (TCE). It is noteworthy that 1,4-dioxane often occurs in symbiosis with 1,1,1-TCA and has a similarity contamination plume distribution to 1,1,1-TCA. The natural attenuation of 1,4-dioxane in groundwater environment is weak, but there is a certain degree of biological oxidation attenuation. Current methods for treating 1,4-dioxane pollution mainly include extraction-treatment technology, advanced oxidation treatment technology, modified biological treatment technology and phytoremediation technology, all of which have their limitations in practical application. Currently, there is no environmental regulation available for the 1,4-dioxane pollution worldwide, and no enforceable standard established for defining the health trigger levels of 1,4-dioxane in drinking water. Research on this contaminant in China is generally limited to the site or laboratory scale, and there are no studies on the environmental risk and quality standards for 1,4-dioxane in the water environment.
-
Key words:
- 1,4-dioxane /
- Chlorinated hydrocarbon /
- Environmental pollution /
- Attenuation
-
Table 1. Drinking water guidelines and criteria for 1,4-dioxane (An et al. 2014; US EPA, 2017b; US EPA, 2018a, US EPA, 2018b; Health Canada, 2018; Mulisch et al. 2003; WHO, 2005; Yamamoto et al. 2018)
Jurisdiction Target concentration
/µg/LType of target Year target was introduced Non-enforced guidance values for 1,4-dioxane in developed countries and WHO Canada 50.0 Health Canada, Draft drinking water guidelines 2018 United States 0.35 US EPA, Screening levels for tap watera 2017 Korea 50.0 Ministry of the environment, Provisional standards 2014 Japan 50.0 Water pollution control Law, Drinking water standards 2009 Germany 0.1 EPA, Recommended guidance 2003 WHO 50.0 Guidance value 2005 Non-mandatory guidance values for 1,4-dioxane by US states New York 1.0 Draft minimum standards 2018 Michigan 7.2 Drinking water standards 2017 Alaska 77.0 Groundwater purification level 2016 Texas 9.1 Protection concentration level 2016 Maine 4.0 Guidance value 2016 Indiana 4.6 Groundwater screening level 2016 North Carolina 3.0 Drinking water standards 2015 New Jersey 0.4 Groundwater quality standards 2015 Connecticut 3.0 Intervention level 2013 Minnesota 1.0 Health risk limits 2013 California 1.0 Public health protection concentration 2011 Massachusetts 0.3 Guidance value 2004 a The US EPA has not set a minimum level of enforceable standards. -
Abe A. 1999. Distribution of 1, 4-dioxane in relation to possible sources in the water environment. Science of the Total Environment, 227(1): 41−47. DOI: 10.1016/S0048-9697(99)00003-0. Adamson DT, Anderson RH, Mahendra S, et al. 2015. Evidence of 1, 4-dioxane attenuation at groundwater sites contaminated with chlorinated solvents and 1, 4-dioxane. Environmental Science & Technology, 49(11): 6510−6518. DOI: 10.1021/acs.est.5b00964. Adamson DT, Blanc PCD, Farhat SK, et al. 2016. Implications of matrix diffusion on 1, 4-dioxane persistence at contaminated groundwater sites. Science of the Total Environment, 562: 98−107. DOI: 10.1016/j.scitotenv.2016.03.211. Adamson DT, Mahendra S, Walker KL, et al. 2014. A multi-site survey to identify the scale of the 1, 4-dioxane problem at contaminated groundwater sites. Environmental Science Technology Letters, 1: 254−258. DOI: 10.1021/ez500092u. Adamson DT, Piña EA, Cartwright AE, et al. 2017. 1, 4-Dioxane drinking water occurrence data from the third unregulated contaminant monitoring rule. Science of the Total Environment, 596–597: 236−245. DOI: 10.1016/j.scitotenv.2017.04.085. Adamson DT, Wilson JT, Freedman DL, et al. 2022. Establishing the prevalence and relative rates of 1, 4-dioxane biodegradation in groundwater to improve remedy evaluations. Journal of Hazardous Materials, 424: 127736. DOI: 10.1016/j.jhazmat.2021.127736. Agency for Toxic Substances and Disease Registry (ATSDR). 2012. Division of toxicology and environmental medicine/Applied toxicology branch, US department of health and human services, 2012. Toxicological Profile for 1: 4−Dioxane. Agency for toxic substances and disease registry (ATSDR). 2019. The ATSDR 2019 Substance Priority List. Alexander E. 2018. Positive trends emerge in reducing exposure to 1, 4-Dioxane. American Water Works Association, 110(7): 54−59. DOI: 10.1002/awwa.1116. Alexander M. 1973. Nonbiodegradable and other recalcitrant molecules. Biotechnology & Bioengineering, 15(4): 611−647. DOI: 10.1002/bit.260150402. An YJ, Kwak J, Nam SH, et al. 2014. Development and implementation of surface water quality standards for protection of human health in Korea. Environmental Science and Pollution Research, 21: 77−85. DOI: 10.1007/s11356-013-1626-9. Anderson RH, Anderson JK, Bower PA. 2012. Co-occurrence of 1, 4-dioxane with trichloroethylene in chlorinated solvent groundwater plumes at US Air Force installations: Fact or fiction. Integrated Environmental Assessment and Management, 8(4): 731−737. DOI: 10.1002/ieam.1306. Barajas-Rodriguez FJ, Murdoch LC, Falta RW, et al. 2019. Simulation of in situ biodegradation of 1, 4-dioxane under metabolic and cometabolic conditions. Journal of Contaminant Hydrology, 223: 103464. DOI: 10.1016/j.jconhyd.2019.02.006. Burmaster DE. 1982. The new pollution: Groundwater contamination. Environment: Science and Policy for Sustainable Development, 24: 33−36. DOI: 10.1080/00139157.1982.9932469. Chiang DS, Glover EWJ, Peterman J, et al. 2008. Evaluation of natural attenuation at a 1,4-dioxane-contaminated site. Remediation Journal, 19(1): 19−37. Chiang SY, Anderson R, Wilken M, et al. 2016. Practical perspectives of 1, 4-Dioxane investigation and remediation. Remediation Journal, 27(1): 7−27. DOI: 10.1002/rem.21494. Daisuke I , Kazuki H, Takuya O, et al. 2020. Carbon sources that enable enrichment of 1, 4-dioxanedegrading bacteria in landfill leachate. Biodegradation, 31(1-2): 23−34. DOI: 10.1007/s10532-019-09891-w. Daisuke I, Takumi Y, Takuya O, et al. 2021. Treatment of 1, 4-dioxane-containing water using carriers immobilized with indigenous microorganisms in landfill leachate treatment sludge: A laboratory-scale reactor study. Journal of Hazardous Materials, 414: 125497. DOI: 10.1016/j.jhazmat.2021.125497. Dawson D, Fisher H, Noble AE, et al. 2022. Assessment of non-Occupational 1, 4-Dioxane exposure pathways from drinking water and product use. Environmental Science & Technology, 56: 5266−5275. DOI: 10.1021/acs.est.1c06996. Ernstgård L, Iregren A, Sjögren B. 2006. Acute effects of exposure to vapours of dioxane in humans. Human & Experimental Toxicology, 25: 723−729. DOI: 10.1177/0960327106073805. European Chemicals Agency (ECHA), 2021. Substance Information, 1, 4-Dioxane. Fei YH,Liu YC,Li YS, et al. 2022. Prospect of groundwater pollution remediation methods and technologies in China. Geology in China, 49(2): 420−434. (in Chinese) DOI: 10.12029/gc20220206. Flis K. 2021. Advanced treatment solutions for 1, 4-Dioxane. American Water Works Association Journal, 113(8): 36−39. DOI: 10.1002/awwa.1785. Fujiwara T, Tamada T, Kurata Y, et al. 2008. Investigation of 1, 4-dioxane originating from incineration residues produced by incineration of municipal solid waste. Chemosphere, 71: 894−901. DOI: 10.1016/j.chemosphere.2007.11.011. Gedalanga PB, Pornwongthong P, Mora R, et al. 2014. Identification of biomarker genes to predict biodegradation of 1, 4-dioxane. Applied and Environmental Microbiology, 80(10): 3209−3218. DOI: 10.1128/aem.04162-13. Health Canada, 2018. 1, 4-Dioxane in drinking water. Guideline Technical Document for Public Consultation. Government of Canada, Federal-Provincial-Territorial Committee on Drinking Water, Ottawa, Ontario. Heather S. 2013. 1, 4-Dioxane and the application of phytoremediation at North Carolina hazardous waste groundwater contaminated sites. Raleigh: North Carolina State University, Environmental Assessment. Huang HL, Shen DS, Li N, et al. 2014. Biodegradation of 1, 4-dioxane by a novel strain and its biodegradation pathway. Water, Air & Soil Pollution, 225(9): 1−11. DOI: 10.1007/s11270-014-2135-2. Huang YR, Shi JH, Tang L. 2001. Determination 1, 4-Dioxane in waste water by GC/MS. Journal of Chinese Mass Spectrometry Society, 22(1): 70−74. (in Chinese) DOI: 10.3969/j.issn.1004-2997.2001.01.012. International Agency for Research on Cancer (IARC). 1999. Re-Evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, 71: 589-602. International Agency for Research on Cancer (IARC). 1987. 1, 4-Dioxane. Monographs Supplement 7, International Agency for Research on Cancer, Lyon, France. Jackson RE, Patterson RJ. 1989. A remedial investigation of an organically polluted outwash aquifer. Ground Water Monitoring and Remediation, 9(3): 119−125. DOI: 10.1111/j.1745-6592.1989.tb01159.x. Karges U, Becker J, Püttmann W. 2018. 1, 4-Dioxane pollution at contaminated groundwater sites in western Germany and its distribution within a TCE plume. Science of the Total Environment, 619–620: 712−720. DOI: 10.1016/j.scitotenv.2017.11.043. Kawata K, Ibaraki T, Tanabe A, et al. 2003. Distribution of 1, 4-dioxane and N, N-dimethylformamide in river water from Niigata, Japan. Bulletin of Environmental Contamination and Toxicology, 70: 876−882. DOI: 10.1007/s00128-003-0064-7. Kraybill HF. 1978. Carcinogenesis induced by contaminants in potable water. Bulletin of the New York Academy of Medicine, 54(4): 413−427. Lee CS, Thanh TL, Kim EJ, et al. 2014. Fabrication of novel oxygen-releasing alginate beads as an efficient oxygen carrier for the enhancement of aerobic bioremediation of 1, 4-dioxane contaminated groundwater. Bioresource Technology, 171: 59−65. DOI: 10.1016/j.biortech.2014.08.039. Lesage S, Jackson RE, Priddle MW, et al. 1990. Occurrence and fate of organic solvent residues in anoxic groundwater at the Gloucester landfill, Canada. Environmental Science and Technology, 24(4): 559−566. DOI: 10.1021/es00074a016. Li CX. 2014. Microbial degradation of 1, 4 – dioxane. M.S. thesis, Beijing: China University of Geosciences (Beijing). (in Chinese). Li F, Deng DY, Zeng LK, et al. 2021. Sequential anaerobic and aerobic bioaugmentation for commingled groundwater contamination of trichloroethene and 1, 4-dioxane. Science of the Total Environment, 774: 145118. DOI: 10.1016/j.scitotenv.2021.145118. Li MY, Orden ETV, DeVries DJ, et al. 2015. Bench-scale biodegradation tests to assess natural attenuation potential of 1, 4-dioxane at three sites in California. Biodegradation, 26(1): 39−50. DOI: 10.1007/s10532-014-9714-1. Liu WH, Medina MA, Thomann W, et al. 2000. Optimization of intermittent pumping schedules for aquifer remediation using a genetic algorithm. Journal of the American Water Resources Association, 36(6): 1335−1348. DOI: 10.1111/j.1752-1688.2000.tb05730.x. Liu X, Xiao JG, Chen W. 2020. Research progress on rRemoval of 1, 4-dioxane from water. Science and Technology Innovation Herald, 31: 94−98. (in Chinese) DOI: 10.16660/j.cnki.1674-098X.2007-5640-2092. Ma XL, Gao S, Song XL, et al. 2015. Progress in the study of technologies of 1, 4-dioxane polluted water treatment. Environmental Protection Science, 41(5): 108−113. (in Chinese) DOI: 10.3969/j.issn.1004-6216.2015.05.020. Mahendra S, Alvarez-Cohen L. 2006. Kinetics of 1, 4-dioxane biodegradation by monooxygenase-expressing bacteria. Environmental Science and Technology, 40(17): 5435−5442. DOI: 10.1021/es060714v. Matsushita T, Sugita W, Ishikawa T, et al. 2019. Prediction of 1, 4-dioxane decomposition during VUV treatment by model simulation taking into account effects of coexisting inorganic ions. Water Research, 164: 114918. DOI: 10.1016/j.watres.2019.114918. McElroy AC, Hyman MR, Knappe DRU. 2019. 1, 4-Dioxane in drinking water emerging for 40 years and still unregulated. Current Opinion in Environmental Science & Health, 7: 117−125. DOI: 10.1016/j.coesh.2019.01.003. Milavec J, Tick GR, Brusseau ML, et al. 2019. 1, 4-Dioxane cosolvency impacts on trichloroethene dissolution and sorption. Environmental Pollution, 252: 777−783. DOI: 10.1016/j.envpol.2019.05.156. Ministry of Environment and Infrastructure. 2015. The Quality of Drinking Water in the Netherlands. The Hague, Netherlands. Mohr TKG. 2001. Solvent Stabilizers. Santa Clara Valley Water District, San Jose, CA. Mohr TKG, Stickney JA, DiGuiseppi WH. 2010. Environmental investigation and remediation: 1, 4-Dioxane and Other Solvent Stabilizers. CRC Press, Taylor and Francis Group. Mulisch HM, Winter W, Dieter HH. 2003. Modulares system zur gesamtbewertung von umweltkontaminanten in Boden, Gewässern und Trinkwasser (Modular system for total evaluation of environmental contaminants in soil and water.). Bundesgesundheitsbl–Gesundheitsforsch–Gesundheitsschutz, 46: 668−676. DOI: 10.1007/s00103-003-0634-1. Myers MA, Johnson NW, Marin EZ, et al. 2018. Abiotic and bioaugmented granular activated carbon for the treatment of 1, 4-dioxane-contaminated water. Environmental Pollution, 240: 916−924. DOI: 10.1016/j.envpol.2018.04.011. Nyer EK, Kramer V, Valkenburg N. 1991. Biochemical effects on contaminant fate and transport. Ground Water Monitoring and Remediation, 11(2): 80–82. Osama R, Ibrahim MG, Elreedy A, et al. 2020. Long-term assessment of 1, 4-dioxane uptake via duckweed with emphasis on operational parameters. Materials Science Forum, 1008: 121−127. DOI: 10.4028/www.scientific.net/MSF.1008.121. Parales RE, Adamus JE, White N, et al. 1994. Degradation of 1, 4-dioxane by an actinomycete in pure culture. Applied and Environmental Microbiology, 60(12): 4527−4530. DOI: 10.1016/0922-338X(95)92742-U. Patterson RJ, Jackson RE, Graham BW, et al. 1985. Retardation of toxic chemicals in a contaminated outwash aquifer. Water Science & Technology, 17(9): 57−69. DOI: 10.1029/WR021i012p01923. Paxe´us N. 2000. Organic compounds in municipal landfill leachates. Water Science & Technology, 42(7): 323−332. DOI: 10.1029/1999WR900289. Pollitt KG, Kim JH, Peccia J, et al. 2019. 1, 4-Dioxane as an emerging water contaminant: State of the science and evaluation of research needs. Science of the Total Environment, 690: 853−866. DOI: 10.1016/j.scitotenv.2019.06.443. Pornwongthong P, Mulchandani A, Gedalanga P B, et al. 2014. Transition metals and organic ligands influence biodegradation of 1, 4-dioxane. Applied Biochemistry and Biotechnology, 173(1): 291−306. DOI: 10.1007/s12010-014-0841-2. Postigo C, Barceló D. 2015. Synthetic organic compounds and their transformation products in groundwater: occurrence, fate and mitigation. Science of the Total Environment, 503–504: 32−47. DOI: 10.1016/j.scitotenv.2014.06.019. Priddle MW, Jackson RE. 1991. Laboratory column measurement of VOC retardation factors and comparison with field values. Ground Water, 29(2): 260−266. DOI: 10.1111/j.1745-6584.1991.tb00518.x. Reierson RL. 1995. Process of making low dioxane alkoxylate phosphate esters. United States Patent 5, 463, 101, assigned to Rhone-Poulenc, Inc., October 31, 1995. Romero J, Ventura F, Caixach J, et al. 1998. Identification of 1, 3-dioxanes and 1, 3 dioxolanes as malodorous compounds at trace levels in river water, groundwater, and tap water. Environmental Science and Technology, 32(2): 206−216. DOI: 10.1021/es9704085. Roy D, Anagnostu G, Chaphalkar P. 1995. Analysis of respirometric data to obtain kinetic coefficients for biodegradation of 1, 4-dioxane. Journal of Environmental Science and Health, 30(8): 1775−1790. DOI: 10.1080/10934529509376301. Roy WR, Griffin RA. 1985. Mobility of organic solvents in water-saturated soil materials. Environmental Geology and Water Sciences, 7: 241−247. DOI: 10.1007/BF02509925. Sales CM, Grostern A, Parales JV, et al. 2013. Oxidation of the cyclic ethers 1, 4-dioxane and tetrahydrofuran by a monooxygenase in two Pseudonocardia species. Applied and Environmental Microbiology, 79(24): 7702−7708. DOI: 10.1128/AEM.02418-13. Sei K, Kakinoki T, Inoue D, et al. 2010. Evaluation of the biodegradation potential of 1, 4-dioxane in river, soil and activated sludge samples. Biodegradation, 21: 585−591. DOI: 10.1007/s10532-010-9326-3. Sei K, Miyagaki K, Kakinoki T, et al. 2013. Isolation and characterization of bacterial strains that have high ability to degrade 1, 4-dioxane as a sole carbon and energy source. Biodegradation, 24(5): 665−674. DOI: 10.1007/s10532-012-9614-1. Sekar R, DiChristina TJ. 2014. Microbially driven fenton reaction for degradation of the widespread environmental contaminant 1, 4-dioxane. Environmental Science and Technology, 48(21): 12858−12867. DOI: 10.1021/es503454a. Shen WR, Chen H, Pan SS. 2008. Anaerobic biodegradation of 1, 4-dioxane by sludge enriched with iron-reducing microorganisms. Bioresource Technology, 99(7): 2483−2487. DOI: 10.1016/j.biortech.2007.04.054. Shen W, Wang Y, Zhan J, et al. 2017. Kinetics and operational parameters for 1, 4-dioxane degradation by the photoelectro-peroxone process. Chemical Engineering Journal, 310: 249−258. DOI: 10.1016/j.cej.2016.10.111. Stepien DK, Diehl P, Helm J, et al. 2014. Fate of 1, 4-dioxane in the aquatic environment: from sewage to drinking water. Water Research, 48: 406−419. DOI: 10.1016/j.watres.2013.09.057. Stepien DK, Püttmann W. 2013. Simultaneous determination of six hydrophilic ethers at trace levels using coconut charcoal adsorbent and gas chromatography/mass spectrometry. Analytical and Bioanalytical Chemistry, 405: 1743−1751. DOI: 10.1007/s00216-012-6571-9. Stickney JA, Sager SL, Clarkson JR, et al. 2003. An updated evaluation of the carcinogenic potential of 1, 4-dioxane. Regulatory Toxicology and Pharmacology, 38: 183−195. DOI: 10.1016/S0273-2300(03)00090-4. Stuart M, Lapworth D, Crane E, et al. 2012. Review of risk from potential emerging contaminants in UK groundwater. Science of the Total Environment, 416: 1−21. DOI: 10.1016/j.scitotenv.2011.11.072. Tanabe A, Tsuchida Y, Ibaraki T, et al. 2006. Impact of 1, 4-dioxane from domestic effluent on the Agano and Shinano Rivers, Japan. Bulletin of Environmental Contamination and Toxicology, 76: 44−51. DOI: 10.1007/s00128-005-0887-5. US EPA. 1995. OPPT Chemical Fact Sheets—1, 4-Dioxane fact sheet. Pollution Prevention and Toxics. Washington, DC: United States Environmental Protection Agency. US EPA. 2009. Fact Sheet: Final Third Drinking Water Contaminant Candidate List (CCL3). US EPA. 2011. Reportable quantities of hazardous substances designated pursuant to section 311 of the clean water act. Code of Federal Regulations. 40 CFR 302.4. Washington, DC. US EPA. 2013. 1, 4-Dioxane (CASRN 123-91-1) – Integrated Risk Information System (IRIS). US EPA. 2017a. Scope of risk evaluation for 1, 4-Dioxane. US EPA. 2017b. Technical Fact Sheet-1, 4-Dioxane. United States Environmental Protection Agency, Washington, DC. US EPA. 2018a. Problem formulation of the risk evaluation. 1, 4-Dioxane CASRN : 123-91-1. US EPA. 2018b. Toxics release inventory (TRI), Reporting Year 2017, Washington, DC. US EPA. 2018c. Edition of the drinking water standards and health advisories tables (EPA 822-F-18-001). US EPA. 2013. Occurrence Data: Accessing unregulated contaminant monitoring data (http://water.epa.gov/lawsregs/rulesregs/sdwa/ucmr/data.cfm#ucmr2013) (accessed April 2021). World Health Organization (WHO). 2005. 1, 4-Dioxane in drinking-water. Background Document for Development of WHO Guidelines for Drinking-water Quality. World Health Organization, Geneva: Switzerland. Xue S, Sun SB, Qing WH, et al. 2021. Experimental and computational assessment of 1, 4-Dioxane degradation in a photo-Fenton reactive ceramic membrane filtration process. Frontiers of Environmental Science & Engineering, 15(5): 95. DOI: 10.1007/s11783-020-1341-y. Yamamoto N, Inoue D, Sei K, et al. 2018. Field test of on-site treatment of 1, 4-dioxane-contaminated groundwater using Pseudonocardia sp. D17. Journal of Water and Environment Technology, 16: 256−268. DOI: 10.2965/jwet.18-033. Yan N, Zhong H, Brusseau ML. 2019. The natural activation ability of subsurface media to promote in-situ chemical oxidation of 1, 4-dioxane. Water Research, 149: 386−393. DOI: 10.1016/j.watres.2018.11.028. Yang Z, Zhou JD, Tang HY. 2021. Research progress of fenton process on 1,4-dioxane treatment. Shandong Chemical Industry, 50(18): 66−67, 69. (in Chinese) DOI: 10.3969/j.issn.1008-021X.2021.18.023. Yasuhara A, Tanaka Y, Tanabe A, et al. 2003. Elution of 1,4-dioxane from waste landfill sites. Bulletin of Environmental Contamination and Toxicology, 71(3): 641−647. DOI: 10.1007/s00128-003-8917-7. Zenker MJ, Borden RC, Barlaz MA. 2002. Modeling cometabolism of cyclic ethers. Environmental Engineering Science, 19(4): 215−228. DOI: 10.1089/109287502760271535. Zenker MJ, Borden RC, Barlaz MA. 2003. Occurrence and treatment of 1,4-dioxane in aqueous environments. Environmental Engineering Science, 20(5): 423−432. DOI: 10.1089/109287503768335913. Zhou DP, Huang YR, Ren J, et al. 2012. Degradation of 1,4-dioxane. Detergent & Cosmetics, 35(5): 16−19. (in Chinese) DOI: 10.3969/j.issn.1006-7264.2012.05.005. Zhou ZQ, Zeng Q, Li GY, et al. 2021. Oxidative degradation of commingled trichloroethylene and 1,4-dioxane by hydroxyl radicals produced upon oxygenation of a reduced clay mineral. Chemosphere, 290: 133265. DOI: 10.1016/j.chemosphere.2021.133265. -

 E-mail alert
E-mail alert Rss
Rss



 下载:
下载: