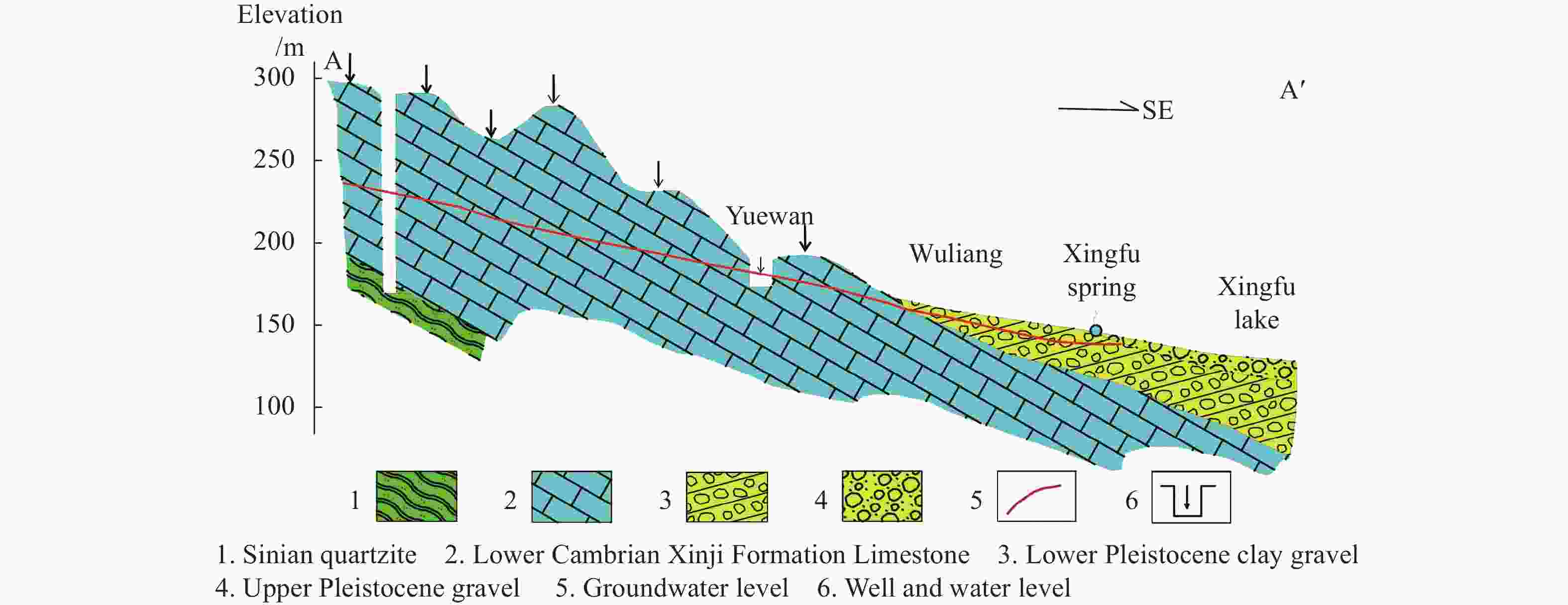
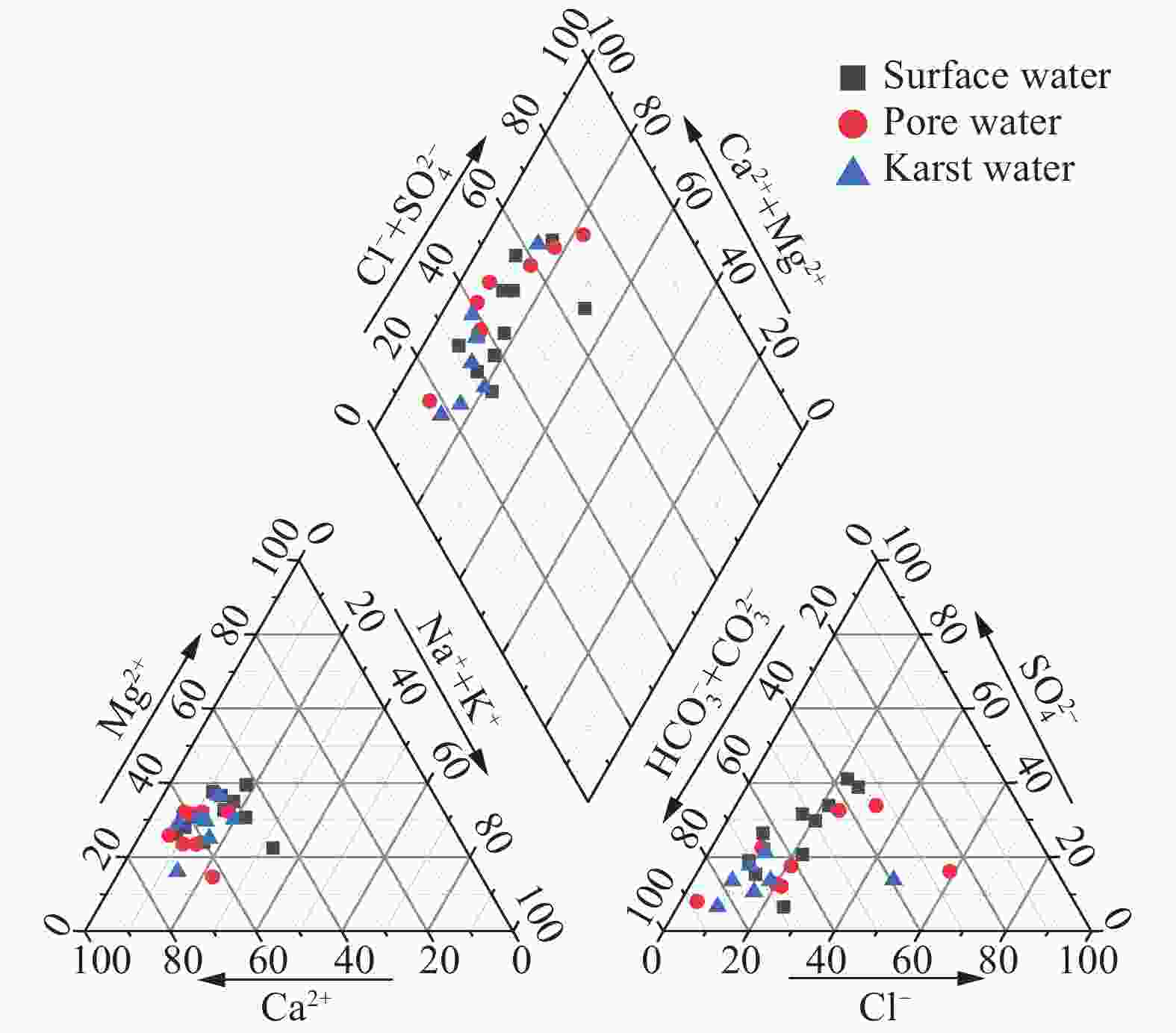
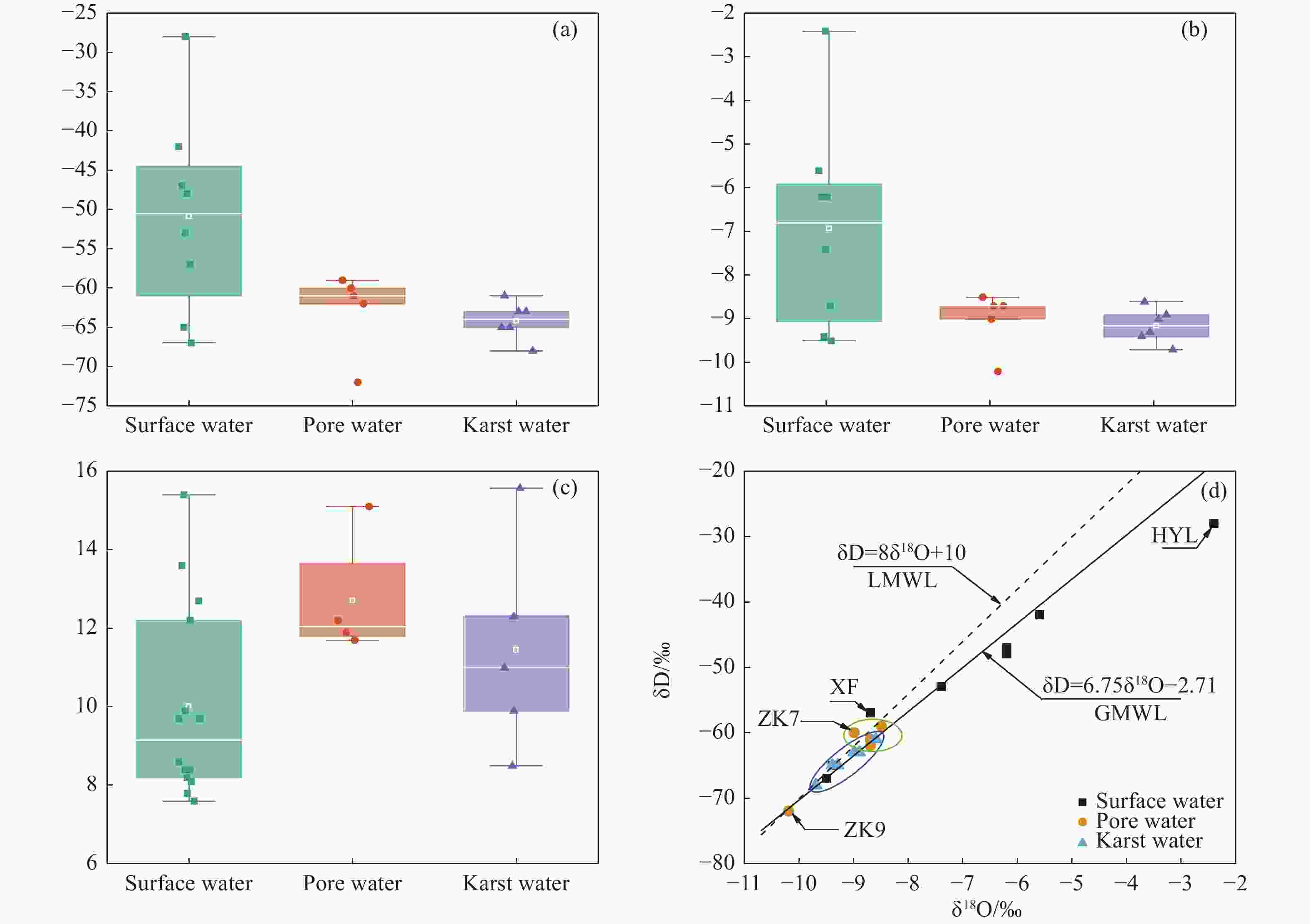
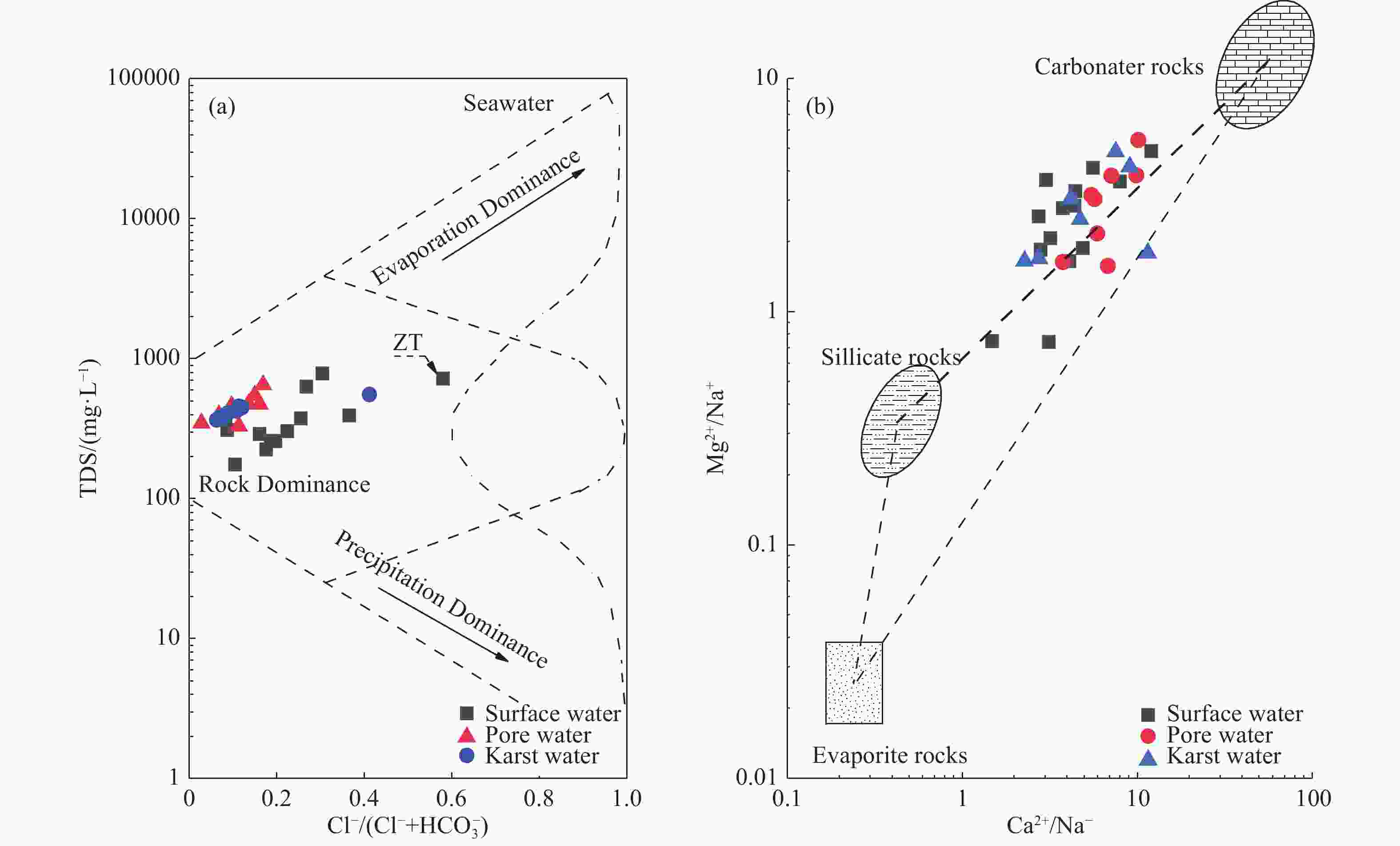
Identified the hydrochemical and the sulfur cycle process in subsidence area of Pingyu mining area using multi-isotopes combined with hydrochemistry methods
-
Abstract: Groundwater serves as an important water source for residents in and around mining areas. To achieve scientific planning and efficient utilization of water resources in mining areas, it is essential to figure out the chemical formation process and the ground water sulfur cycle that transpire after the coal mining activities. Based on studies of hydrochemistry and D,18O-H2O,34S-SO4 isotopes, this study applied principal component analysis, ion ratio and other methods in its attempts to reveal the hydrogeochemical action and sulfur cycle in the subsidence area of Pingyu mining area. The study discovered that, in the studied area, precipitation provides the major supply of groundwater and the main water chemistry effects are dominated by oxidation dissolution of sulfide minerals as well as the dissolution of carbonate and silicate rocks. The sulfate in groundwater primarily originates from oxidation and dissolution of sulfide minerals in coal-bearing strata and human activities. The mixed sulfate formed by the oxidation of sulfide minerals and by human activities continuously recharges the groundwater, promoting the dissolution of carbonate rock and silicate rock in the process.
-
Key words:
- PCA /
- Ion ratio /
- Water chemistry /
- Sulfide minerals /
- Multi-isotopes /
- Subsidence area of mining area
-
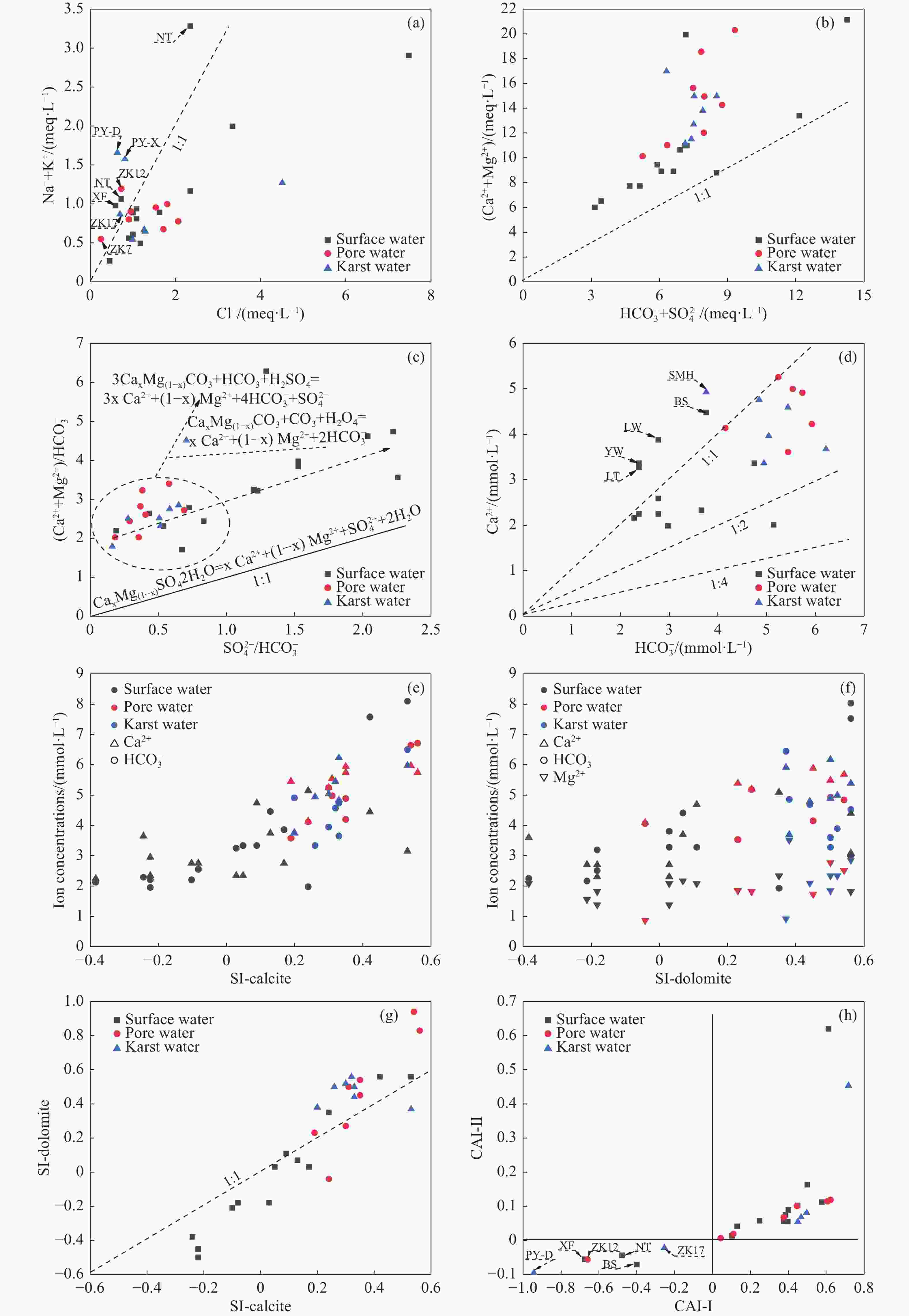
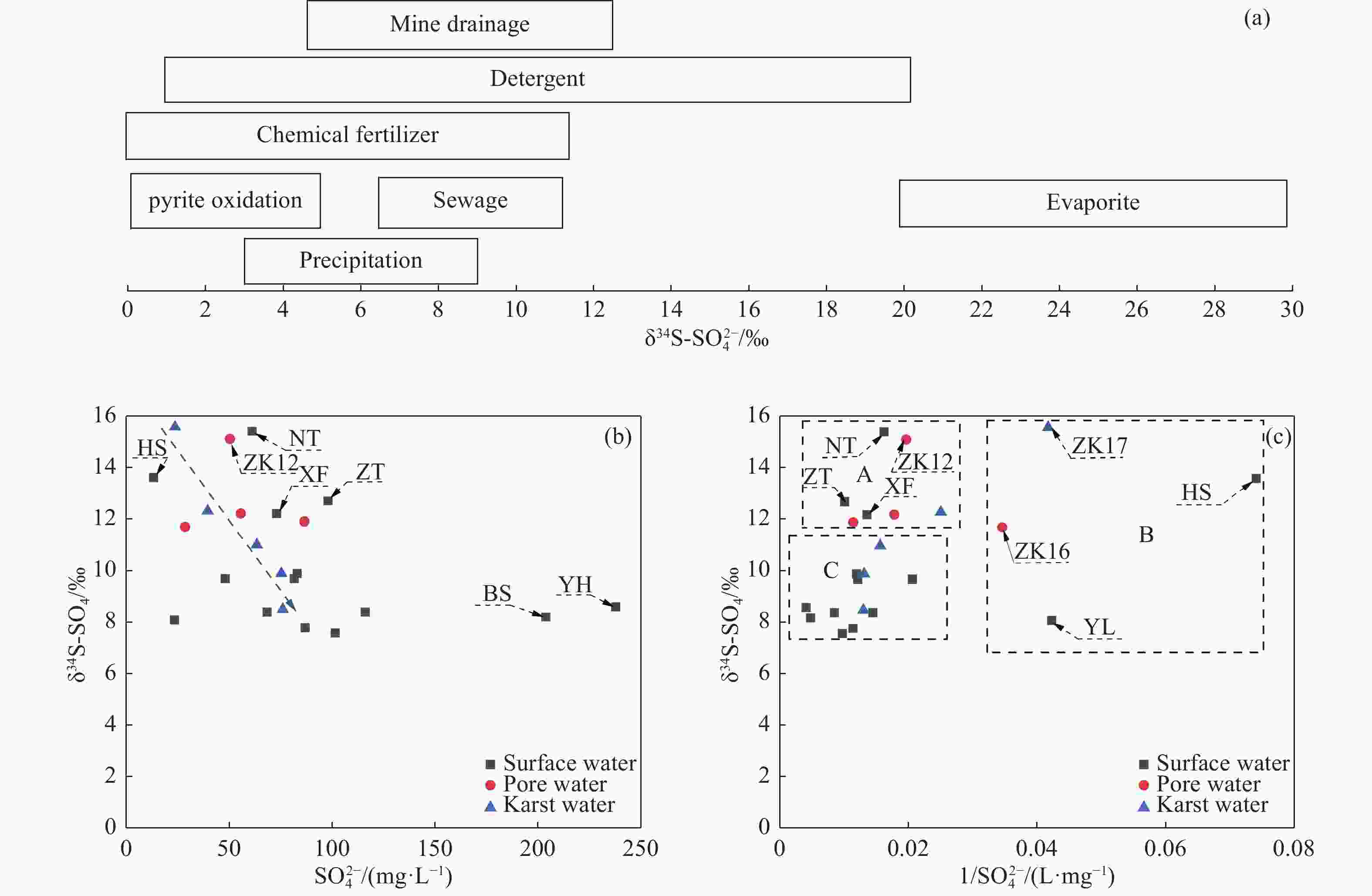
Table 1. Hydrochemical parameters and PHREEQC calculation results of water samples in the study area
Type Number pH K+ Na+ Ca2+ Mg2+ Cl− SO42− HCO3− NO3− H2SiO3 TDS Depth SI-
calciteSI-
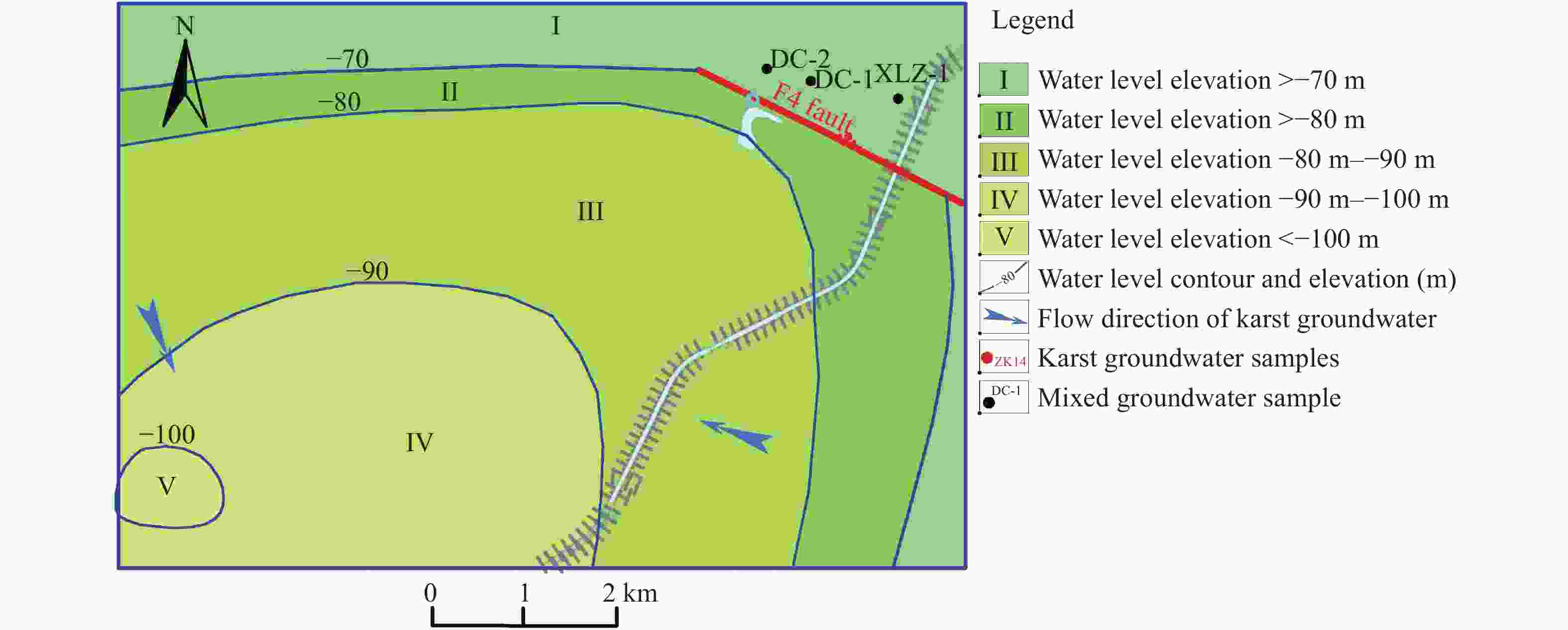
dolomiteConcentration(mg/L) m Surface water XF 7.15 5.45 19.35 46.53 26.13 20.74 73.20 223.62 6.86 20.12 310.64 0.00 −0.24 −0.38 YL 7.19 3.70 4.07 43.09 10.45 15.95 23.65 139.00 4.86 7.44 175.55 0.00 −0.38 −1.02 NT 7.22 12.00 17.44 67.21 26.13 25.52 61.28 290.10 12.70 24.49 368.10 0.00 0.09 0.11 XS 7.25 9.12 15.10 86.17 29.26 35.10 83.16 314.27 16.55 5.36 432.17 0.00 0.24 0.35 HS 7.28 9.38 16.10 39.64 15.68 38.29 13.50 181.31 1.30 5.89 225.28 0.00 −0.22 −0.50 BS 7.30 11.17 68.88 89.62 27.17 82.95 203.80 229.66 34.81 3.30 634.39 0.00 0.13 0.07 YH 7.33 6.56 42.06 151.66 36.58 118.05 237.70 271.96 53.42 16.48 782.56 0.00 0.42 0.56 HTL 7.35 4.36 11.46 44.81 19.86 35.10 68.60 145.05 1.29 6.41 258.43 0.00 −0.22 −0.45 MG 7.37 4.05 10.54 51.70 22.99 31.91 81.72 169.22 1.04 3.04 289.07 0.00 −0.08 −0.18 LFS 7.40 8.74 13.55 44.81 19.86 38.29 48.26 169.22 1.09 1.24 259.60 0.00 −0.10 −0.21 LT 7.45 3.51 9.33 65.49 17.77 41.48 87.04 145.05 6.00 0.20 303.63 0.00 0.03 −0.18 LW 7.47 4.32 18.02 77.55 17.77 57.43 101.60 169.22 13.60 0.20 375.37 0.00 0.17 0.03 YW 7.49 4.89 24.01 67.21 26.13 82.95 116.20 145.05 <0.88 0.20 394.33 0.00 0.05 0.03 ZT 7.52 13.42 58.90 162.00 22.99 264.81 98.12 193.40 5.38 10.93 723.37 0.00 0.53 0.56 Pore water ZK3 7.23 3.84 20.71 99.96 34.49 63.81 48.84 338.45 31.15 19.14 472.64 55.00 0.31 0.50 ZK2 7.35 4.97 14.92 133.07 42.78 72.99 82.75 364.34 94.25 30.71 651.66 55.00 0.54 0.94 ZK7 7.22 1.89 11.54 72.14 23.33 8.69 23.68 332.66 22.30 22.48 347.62 100.00 0.19 0.23 ZK9 7.36 0.13 15.44 134.43 31.35 60.62 52.66 350.53 76.20 23.24 546.47 70.00 0.56 0.83 ZK11 7.22 1.10 20.18 105.13 22.99 33.50 86.68 320.31 31.15 18.19 461.31 200.00 0.30 0.27 ZK13 7.26 4.08 19.57 98.24 31.35 54.24 55.82 350.53 37.00 17.45 475.93 190.33 0.35 0.54 ZK12 7.29 3.48 25.48 84.45 21.95 25.52 50.60 362.62 3.51 21.16 396.74 132.06 0.35 0.45 ZK16 7.32 7.77 13.87 82.73 11.50 31.91 28.83 253.83 26.68 14.59 330.69 241.00 0.24 −0.04 Karstic water SMH 7.30 3.94 26.90 98.56 43.56 159.53 63.60 229.66 43.50 21.98 554.76 300.00 0.20 0.38 MZ-1 7.32 0.99 11.93 95.22 26.34 35.10 75.55 296.14 28.71 16.12 422.23 400.00 0.33 0.44 ZK17 7.30 3.89 17.72 73.50 23.30 24.57 23.95 380.18 8.44 18.50 365.84 371.00 0.33 0.50 PY-X 7.35 5.81 32.84 79.28 29.26 28.71 61.30 308.23 14.96 25.81 406.79 800.00 0.30 0.52 PY-D 7.38 7.77 33.58 67.21 29.26 22.33 61.32 302.18 7.98 27.63 381.23 800.00 0.26 0.50 ZK19 7.30 3.38 12.90 130.30 12.16 45.62 39.76 364.34 31.12 19.45 458.02 259.80 0.53 0.37 DC-3 7.28 2.73 13.84 91.88 35.46 44.67 76.20 332.40 17.96 21.61 449.33 300.00 0.32 0.56 Table 2. Hydrochemical types in Pingyu Mining area
Type Hydrochemical type Amount Proportion(%) Pore water HCO3—Ca·Mg 6 42.86 HCO3·SO4—Ca·Mg 4 28.57 SO4·HCO3—Ca 1 7.14 SO4·HCO3—Ca·Na 1 7.14 SO4·HCO3·Cl—Ca·Mg 1 7.14 Cl—Ca 1 7.14 Pore water HCO3—Ca·Mg 6 75.00 HCO3—Ca 2 25.00 Karstic water HCO3—Ca·Mg 5 71.43 HCO3—Ca 1 14.29 Cl·HCO3—Ca·Mg 1 14.29 Table 3. Correlation analysis table
K+ Na+ Ca2+ Mg2+ Cl− SO42− HCO3− NO3− H2SiO3 TDS K+ 1.000 Na+ 0.237 1.000 Ca2+ −0.377 0.341 1.000 Mg2+ −0.116 0.342 0.277 1.000 Cl− −0.136 0.474 0.548 0.629 1.000 SO42− −0.266 0.386 0.628 0.509 0.347 1.000 HCO3− −0.285 −0.084 0.439 −0.036 −0.314 0.255 1.000 NO3− −0.297 0.253 0.886 0.513 0.604 0.589 0.311 1.000 H2SiO3 0.079 0.336 0.390 0.438 0.143 0.494 0.378 0.446 1.000 TDS −0.306 0.450 0.923 0.602 0.705 0.734 0.337 0.930 0.513 1.000 Table 4. Eigenvectors of the 3 PCs.
1 2 3 K+ −0.304 −0.130 0.812 Na+ 0.520 −0.023 0.617 Ca2+ 0.793 0.414 −0.226 Mg2+ 0.723 −0.063 0.229 Cl− 0.872 −0.399 0.052 SO42− 0.688 0.392 0.070 HCO3− 0.021 0.902 −0.228 NO3− 0.850 0.310 −0.158 H2SiO3 0.391 0.628 0.467 TDS 0.936 0.321 −0.045 Total 4.502 1.912 1.448 Variance percentage (%) 45.016 19.121 14.482 Accumulate% 45.016 64.137 78.620 -
Acharya BS, Kharel G. 2020. Acid mine drainage from coal mining in the United States - An overview. Journal of Hydrology, 588: 125061. DOI: 10.1016/j.jhydrol.2020.125061. Ansari MA, Noble J, Deodhar A, et al. 2020. Atmospheric factors controlling the stable isotopes (δ18O and δ2H) of the Indian summer monsoon precipitation in a drying region of Eastern India. Journal of Hydrology, 584: 124636. DOI: 10.1016/j.jhydrol.2020.124636. Ágnes Ó, Juarez AF, Mariette S, et al. 2022. Sulfur and oxygen isotope constraints on sulfate sources and neutral rock drainage-related processes at a South African colliery. Science of the Total Environment, 846: 157178. DOI: 10.1016/j.scitotenv.2022.157178. Banks D, Boyce AJ, Burnside NM, et al. 2020. On the common occurrence of sulphate with elevated δ34S in European Mine waters: Sulphides, evaporites or seawater? International Journal of Coal Geology, 232: 103619. Cha XF, Wu P, Li XX, et al. 2022. Karst hydrogeochemical characteristics and controlling factors of carlin-type gold mining area based on hydrochemistry and sulfur isotope. Environmental Science, 43(11): 5084−5095. (in Chinese) DOI: 10.13227/j.hjkx.202112141. David BW, Adrian JB, David B, et al. 2023. The occurrence of elevated δ34S in dissolved sulfate in a multi-level coal mine water system, Glasgow, UK. International Journal of Coal Geology, 272: 104248. DOI: 10.1016/j.coal.2023.104248. Gibbs RJ. 1970. Mechanisms controlling world water chemistry. Science, 170(3962): 1088−1090. DOI: 10.1126/science.170.3962.1088. Huang PH, Zhang YN, Li YM, et al. 2023. A multiple isotope (S, H, O and C) approach to estimate sulfate increasing mechanism of groundwater in coal mine area. Science of the Total Environment, 900: 165852. DOI: 10.1016/j.scitotenv.2023.165852. Jacob A, Eric OA, Cynthia L, et al. 2023. Statistical and isotopic analysis of sources and evolution of groundwater. Physics And Chemistry Earth, Parts A/B/C, 129: 103337. Jiang CL, Cheng LL, Li C, et al. 2022. A hydrochemical and multi-isotopic study of groundwater sulfate origin and contribution in the coal mining area. Ecotoxicology and Environmental Safety, 248: 114286. DOI: 10.1016/j.ecoenv.2022.114286. Liu F, Wang S, Yeh TC J, et al. 2020. Using multivariate statistical techniques and geochemical modelling to identify factors controlling the evolution of groundwater chemistry in a typical transitional area between Taihang Mountains and North China Plain. Hydrological Processes, 34(8): 1888−1905. DOI: 10.1002/hyp.13701. Mao HR, Wang CY, Qu S, et al. 2023. Source and evolution of sulfate in the multi-layer groundwater system in an abandoned mine−insight from stable isotopes and Bayesian isotope mixing model. Science of the Total Environment, 859: 160368. DOI: 10.1016/j.scitotenv.2022.160368. Moya CE, Raiber M, Taulis M, et al. 2016. Using environmental isotopes and dissolved methane concentrations to constrain hydrochemical processes and inter-aquifer mixing in the Galilee and Eromanga Basins, Great Artesian Basin, Australia. Journal of Hydrology, 539: 304−318. DOI: 10.1016/j.jhydrol.2016.05.016. Pan GY, Zhang K, Wang PL. 2011. Using stable isotopes to determine the source of mine water supply - Taking Pingyu No. 1 Mine as an Example. Journal of Water Resources and Water Engineering, 22(06): 119−122. (in Chinese) Qu S, Duan LM, Shi ZM, et al. 2022. Hydrochemical assessments and driving forces of groundwater quality and potential health risks of sulfate in a coalfield, northern Ordos Basin, China. Science of the Total Environment, 835: 155519. DOI: 10.1016/j.scitotenv.2022.155519. Ren K, Zeng J, Liang JP, et al. 2021. Impacts of acid mine drainage on Karst aquifers: Evidence from hydrogeochemistry, stable sulfur and oxygen isotopes. Science of the Total Environment, 761: 143223. DOI: 10.1016/j.scitotenv.2020.143223. Rinder T, Dietzel M, Stammeier JA, et al. 2020. Geochemistry of coal mine drainage, groundwater, and brines from the Ibbenbüren Mine, Germany: A coupled elemental-isotopic approach. Applied Geochemistry, 121: 104693. DOI: 10.1016/j.apgeochem.2020.104693. Sabina JK, Kinga S. 2022. Isotopic signature of anthropogenic sources of groundwater contamination with sulfate and its application to groundwater in a heavily urbanized and industrialized area (Upper Silesia, Poland). Journal of Hydrology, 612: 128255. DOI: 10.1016/j.jhydrol.2022.128255. Sahoo S, Khaoash S. 2020. Impact assessment of coal mining on groundwater chemistry and its quality from Brajrajnagar coal mining area using indexing models. Journal of Geochemical Exploration, 215: 106559. DOI: 10.1016/j.gexplo.2020.106559. Su C, Cheng ZS, Wei W, et al. 2018. Assessing groundwater availability and the response of the groundwater system to intensive exploitation in the North China Plain by analysis of long-term isotopic tracer data. Hydrogeology Journal, 26(5): 1401−1415. DOI: 10.1007/s10040-018-1761-y. Su HM, Zhang FW, Hou SY, et al. 2023. An analysis of groundwater circulation in the Pingyu mining area based on hydrochemical and isotopic characteristics of groundwater. Hydrogeology& Engineering Geology, 2023,50(05): 53−67. (in Chinese) DOI: 10.16030/j.cnki.issn.1000-3665.202306010. Tang H. 2011. Numerical simulation and prediction of Karst water dewatering flow in Pingyu 1st coal mine. Henan Province. M. S. thesis. Jiaozuo: Henan Polytechnic University. (in Chinese) Tao M, Cheng WQ, Nie KM, et al. 2022. Life cycle assessment of underground coal mining in China. Science of the Total Environment, 805: 150231. DOI: 10.1016/j.scitotenv.2021.150231. Wang CY, Liao F, Wang GC, et al. 2023. Hydrogeochemical evolution induced by long-term mining activities in a multi-aquifer system in the mining area. Science of the Total Environment, 854: 158806. DOI: 10.1016/j.scitotenv.2022.158806. Wu XX, Chen FL, Zhou X, et al. 2022. Comparative analysis of precipitation isotopes and water vapor sources in Zhengzhou and Fuzhou. Environmental Chemistry, 41(1): 125−134. (in Chinese) Zhang FY. 2017. Division and isotope correlation study of groundwater aquifer group in Xuchang. Ground Water, 39(4): 40−41,111. (in Chinese) DOI: 10.3969/j.issn.1004-1184.2017.04.012. Zhang J, Chen LW, Hou XW, et al. 2021. Multi-isotopes and hydrochemistry combined to reveal the major factors affecting Carboniferous groundwater evolution in the Huaibei Coalfield, North China. Science of the Total Environment, 791: 148420. DOI: 10.1016/j.scitotenv.2021.148420. Zheng LG, Chen X, Dong XL, et al. 2019. Using δ34S–SO4 and δ18O–SO4 to trace the sources of sulfate in different types of surface water from the Linhuan coal-mining subsidence area of Huaibei, China. Ecotoxicology and Environmental Safety, 181: 231−240. DOI: 10.1016/j.ecoenv.2019.06.001. Zhou JW, Zhang YP, Zhou AG, et al. 2016. Application of hydrochemistry and stable isotopes (δ34S, δ18O and δ37Cl) to trace natural and anthropogenic influences on the quality of groundwater in the piedmont region, Shijiazhuang, China. Applied Geochemistry, 71: 63−72. DOI: 10.1016/j.apgeochem.2016.05.018. Zou S, Zhang D, Li XQ, et al. 2022. Sources and pollution pathways of deep groundwater sulfate underneath the Piedmont Plain in the North Henan Province. Earth Science, 47(2): 700−716. (in Chinese) -
 E-mail alert
E-mail alert Rss
Rss



 下载:
下载: