Removal of 1,2,3-Trichloropropane from groundwater using Graphene Oxide-Modified Nano Zero-Valent Iron Activated Persulfate
-
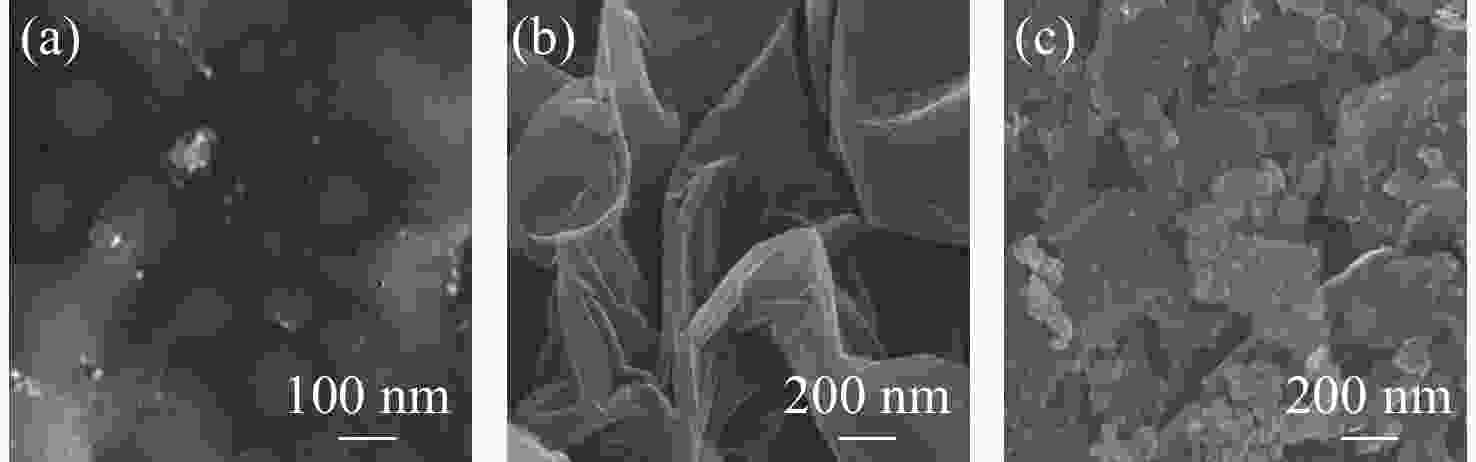
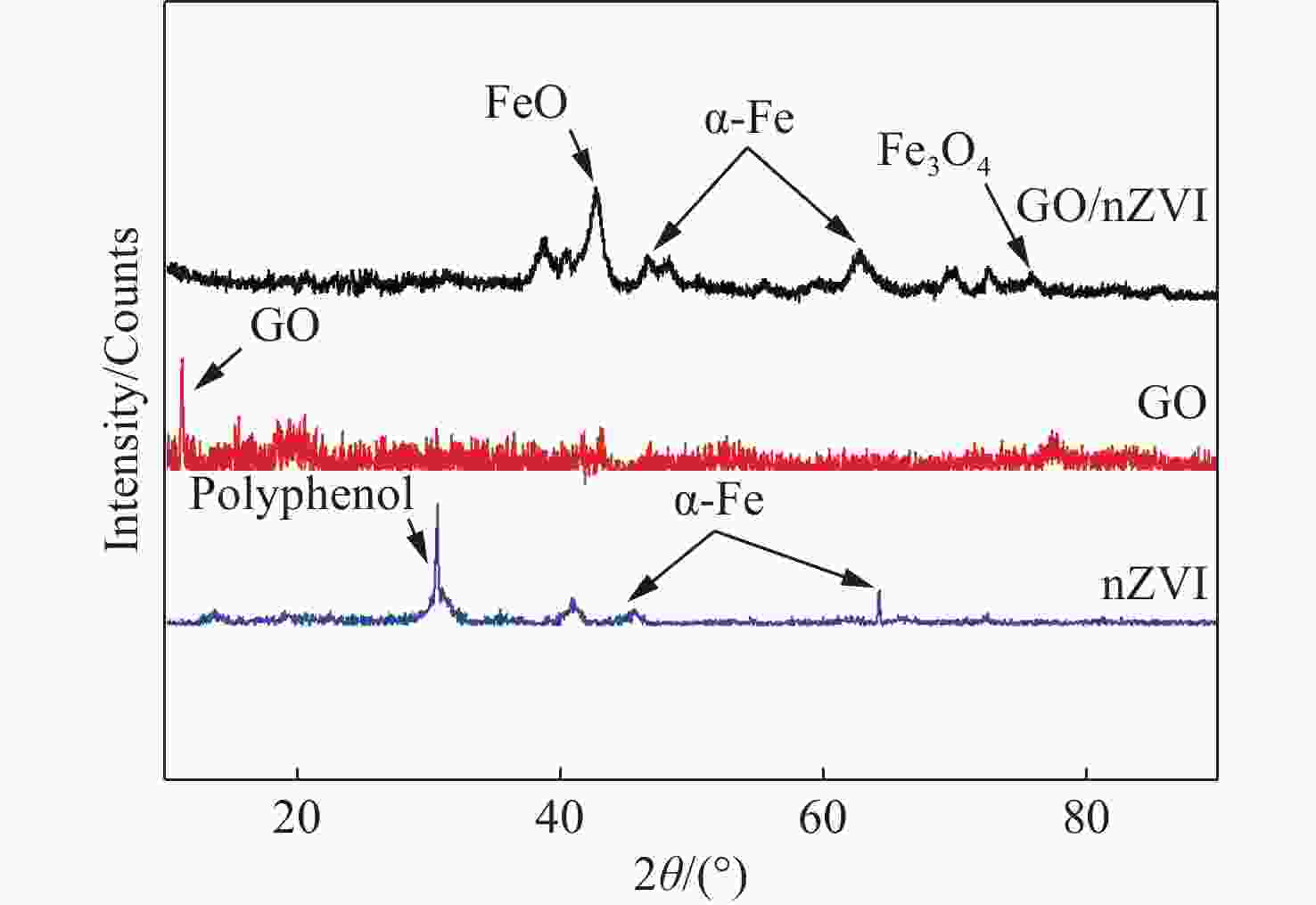
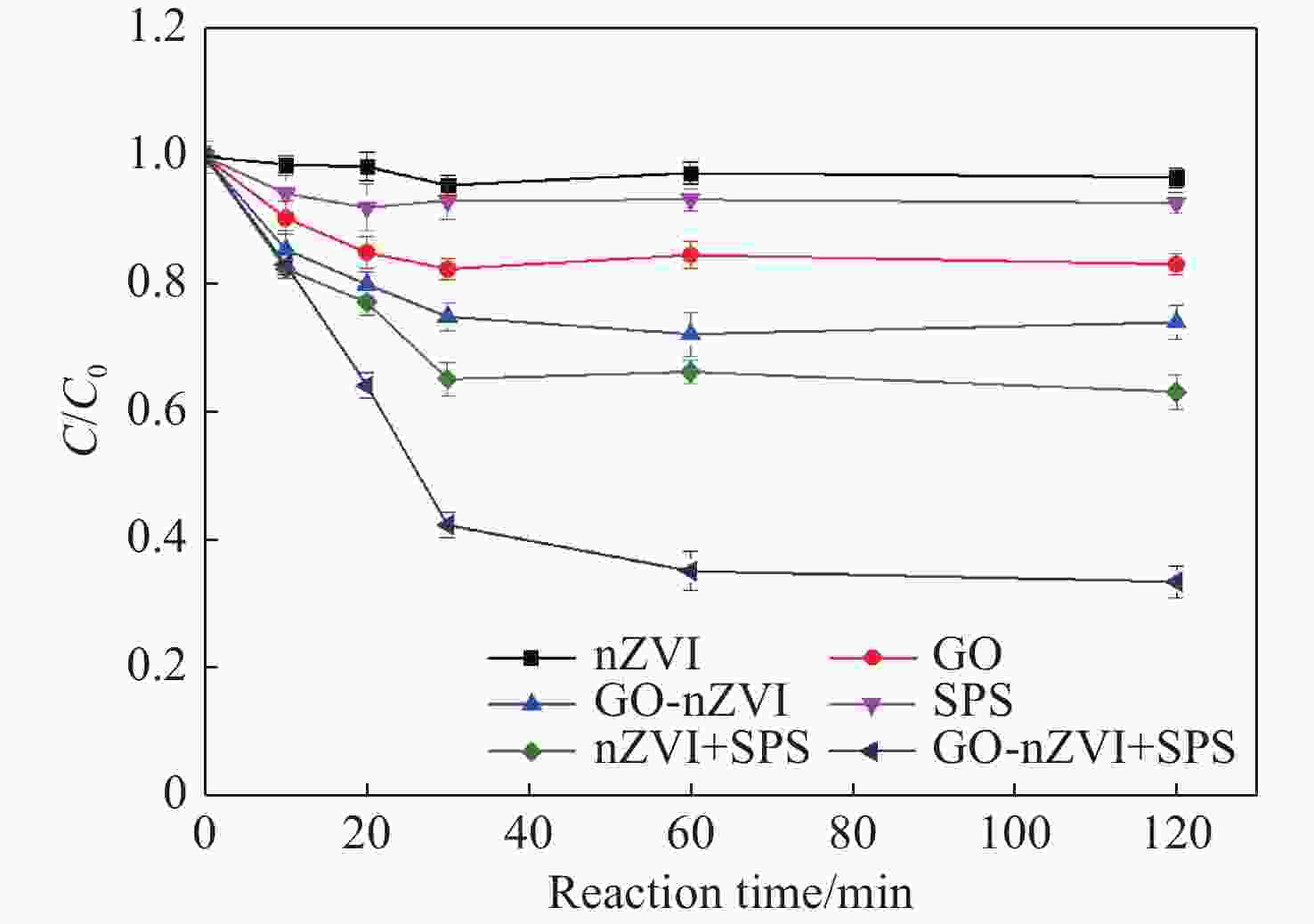
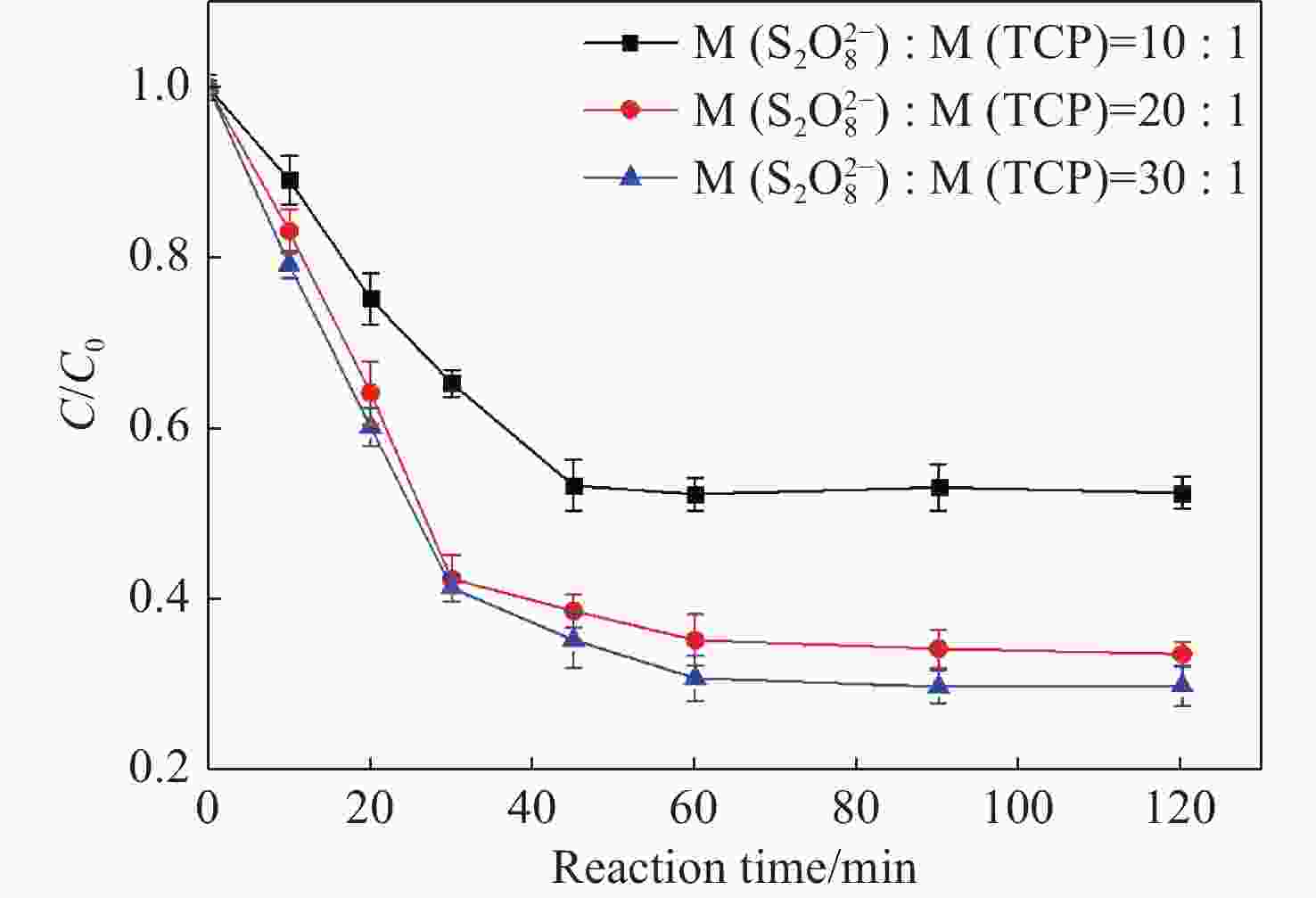
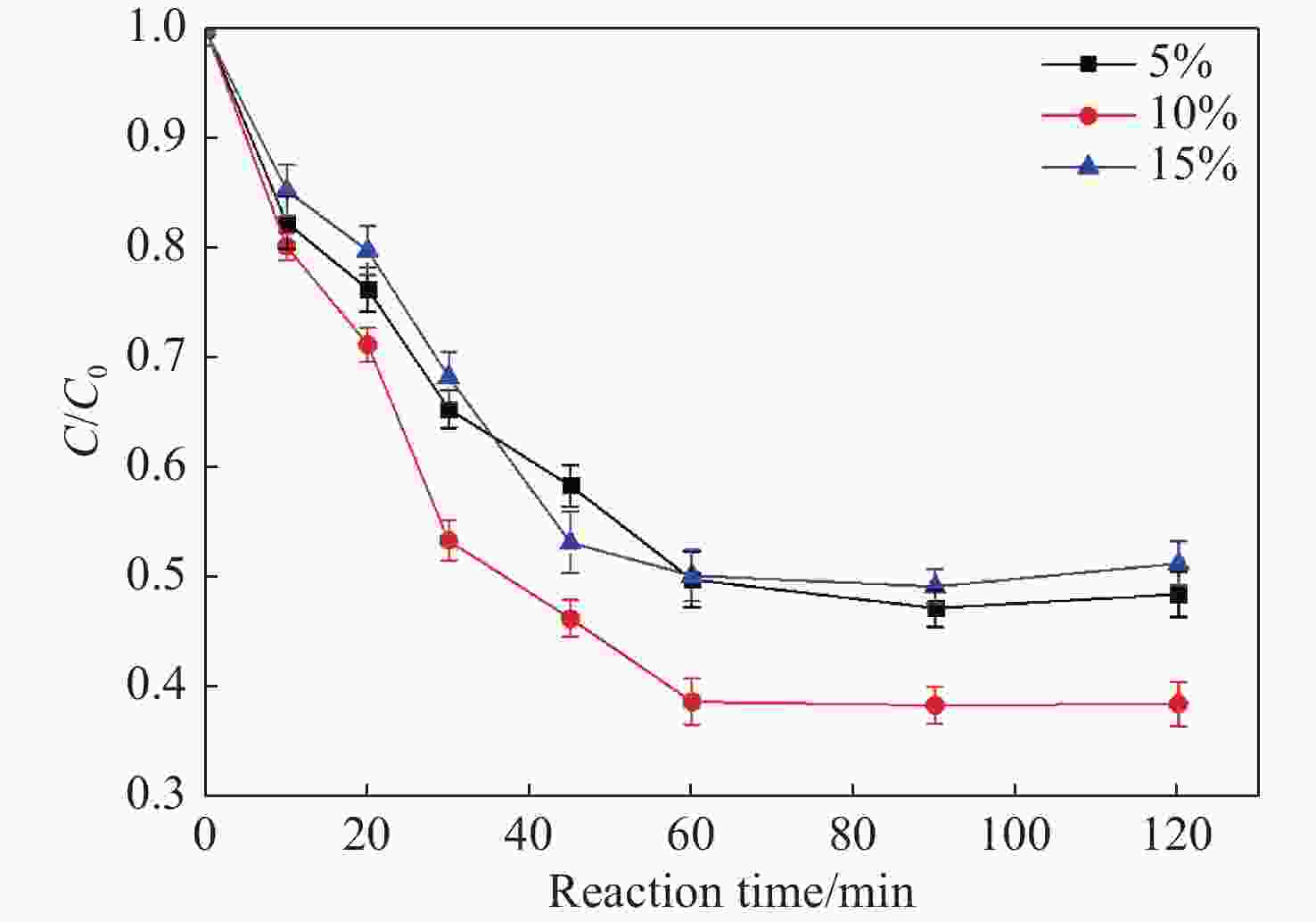
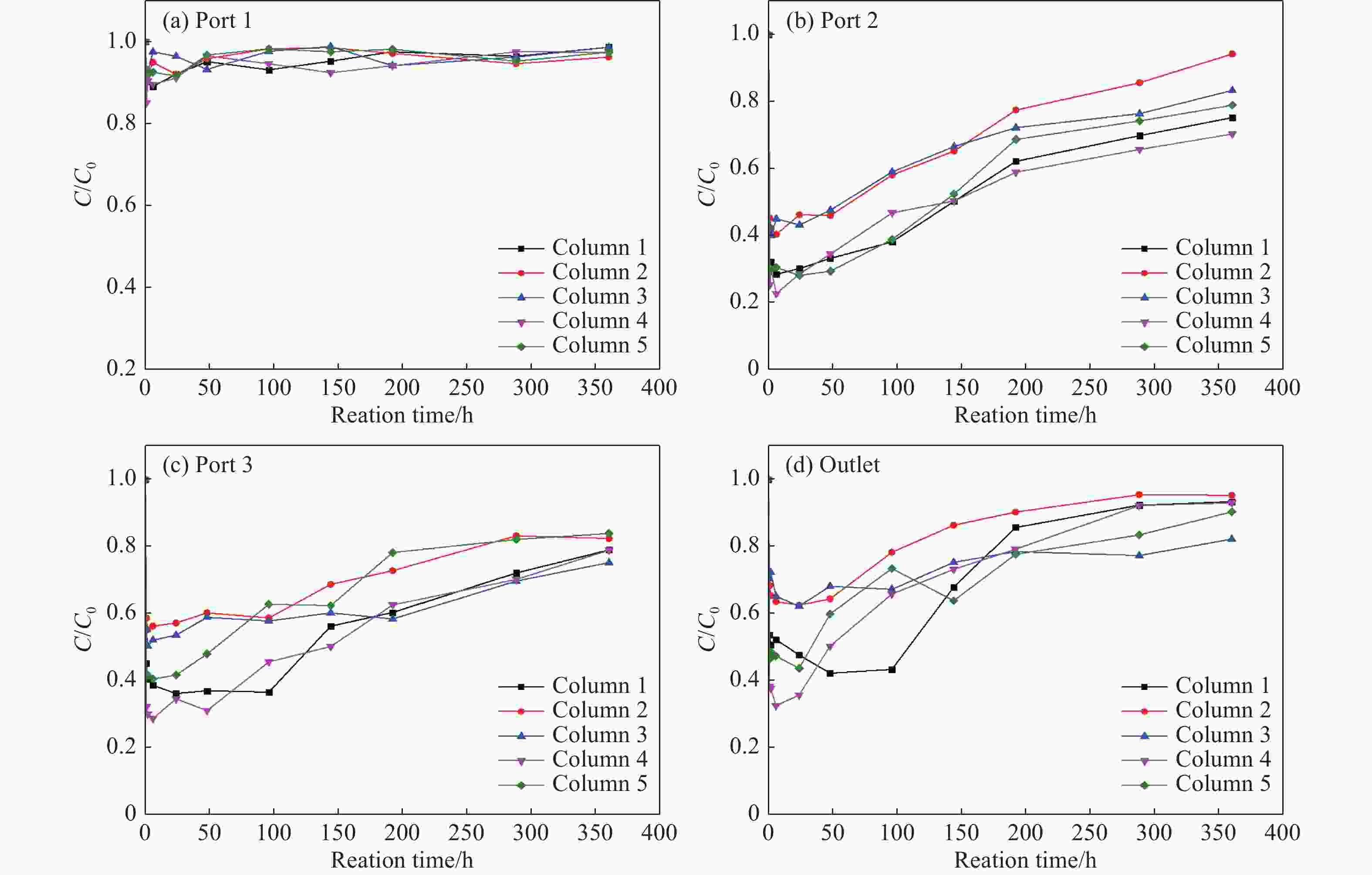
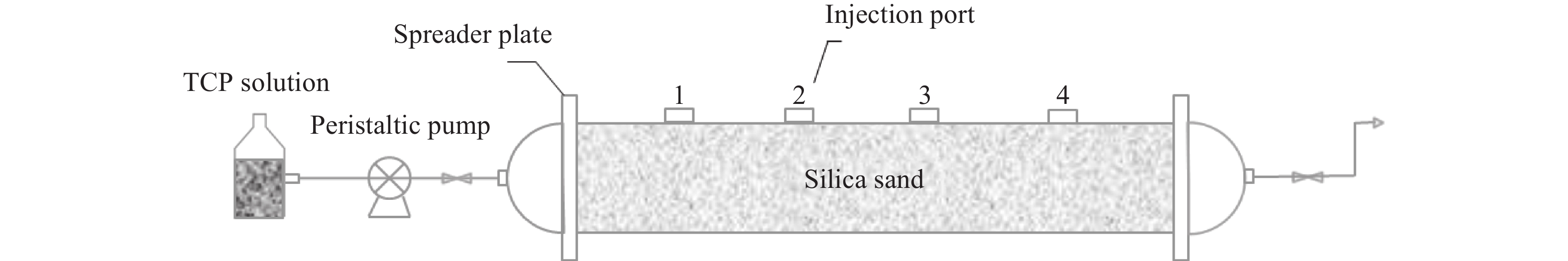
Abstract: Graphene Oxide (GO), nanoscale Zero-Valent Iron (nZVI) and GO-modified nZVI (GO-nZVI) composite materials were prepared by the Hummer and polyphenol reduction method, respectively, and Scanning Electron Microscope (SEM) and X-ray Diffraction (XRD) were used to characterize the morphology and phase composition of these materials. A series of batch experiments were then conducted to investigate the performance and influencing factors of GO-nZVI activating peroxydisulfate (SPS) for the degradation of 1,2,3-trichloropropane (TCP). Finally, an in-situ oxidation reaction zone was created by GO-nZVI-activated SPS in a one-dimensional simulated system to study the remediation of TCP contamination under different aquifer conditions. The results showed that the GO-nZVI composite exhibited a porous, fluffy structure, with spherical nZVI particles loaded onto the surface and folds of the GO sheets. Compared with unmodified nZVI particles, the GO-nZVI composite significantly enhanced the removal efficiency of TCP by activated SPS, achieving a removal rate of 67.2% within an hour - 78.2% higher than that of the unmodified system. The SPS dosage and the C/Fe ratio in GO-nZVI were found to significantly affect the degradation efficiency of TCP. The removal rate of TCP increased with higher SPS concentration, and a 10% carbon addition, yielded the best activation effect. The one-dimensional simulation results indicated that the removal rate of TCP ranged from 30.1% to 73.3% under different conditions. A larger medium particle size and higher concentrations of reactants (SPS and GO-nZVI) improved pollutant degradation efficiency, increasing TCP removal by 62.1%, 23.8%, and 3.7%, respectively. In contrast, a higher groundwater flow velocity was not conducive to the removal of pollutants, with the TCP removal rate decreasing by approximately 41.9%.
-
Table 1. Parameters of test water(mg/L)
pH Na+ Ca2+ Mg2+ SO42− Cl− HCO3− NO3− Fe 6.5 30.50 50.20 42.17 120.45 105.30 88.27 1.53 0.04 Table 2. Parameters of simulated column tests
Columns Medium size /mm Velocity of groundwater
flow /m/dConcentration of SPS /g/L Concentration of GO-NZVI /g/L 1 0.5–1 0.12 35 10 2 0.1–0.25 0.12 35 10 3 0.5–1 0.25 35 10 4 0.5–1 0.12 50 10 5 0.5–1 0.12 35 20 -
Arslan-Alaton I, Koba-Ucun O. 2023. Treatment of reactive dye hydrolysates with UV-C- and ozone-activated percarbonate and persulfate. International Journal of Environmental Research, 17(6): 58−73. DOI: 10.1007/s41742-023-00548-4. Boyandin AN, Bessonnva VA, Sukhanova AA, et al. 2024. Mechanism of thermochemical degradation of bacterial poly-3-hydroxybutyrate in solutions. Russian Chemical Bulletin, 73(3): 688−694. DOI: 10.1007/s11172-024-4179-9. Braki ZA, Sohrabi MR, Motiee F. 2024. Application of central composite design for the simultaneous removal of tetracycline and cefixime using nanoscale zero-valent iron modified by montmorillonite and graphene oxide. Chemical Papers, 78(15): 8181−8193. DOI: 10.1007/s11696-024-03659-0. Chen YT, Qie HT, Zhang YJ, et al. 2020. Synthesis of reduced graphene oxide supported zero-valent iron and its treatment of TNT wastewater. Chemical Journal of Chinese University, 41(8): 1836−1842. DOI: 10.7503/cjcu20200198. Dai XL, Wang S, Li JB, et al. 2023. Reaearch progress on remediation technology of 1, 2, 3-trichloropropane contaminated groundwater. Applied Chemical Industry, 52(4): 1213−1224. DOI: 10.16581/j.cnki.issn1671-3206.2023.04.026. Deng Q, Li H, Han ZT, et al. 2019. Study on the removal efficiency of nano-sized iron particles produced by green tea reduction on Cr(VI) in simulated groundwater. South-North Water Transfers and Water Science & Technology, (1): 130−137. DOI: 10.13476/j.cnki.nsbdqk.2019.0018. Dombek T, Davis D, Stine J, et a1. 2004. Degradation of terbutylazine (2-chloro-4-ethylamino-6-terbutylamino-1, 3, 5-triazine) deisopropyl atrazine (2-amino-4-chloro-6-ethylamino-1, 3, 5-triazine) and chlorinated dimethoxy triazine (2-chloro-4, 6-dimethoxy-1, 3, 5-triazine) by zero valent iron and electrochemical reduction. Environmental Pollution, 129(6): 267−275. DOI: 10.1016/j.envpol.2003.10.008. Horlu M, Macit CK, Aksakal B, et al. 2024. Investigation of the effect of graphene oxide nanoparticles on the structural and dielectric parameters in zinc oxide semiconductors. Journal of Sol-Gel Science and Technology, 110(1): 169−182. DOI: 10.1007/s10971-024-06350-8. Huang XZ, Zhang YX, Zhang DS, et al. 2023. Reduced graphene oxide supported Fe/Ni nanocomposites for 2, 4-dichlorophenol removal. China Environmental Science, 43(12): 6352−6362. DOI: 10.19674/j.cnki.issn1000-6923.2023.0207. Jiang Y, Lu R, Chen Y, et al. 2024. Effect of Fe2+-activated persulfate combined with biodegradation in removing gasoline BTX from karst groundwater: A box-column experimental study. Environmental Science and Pollution Research, 31(28): 50733−50745. DOI: 10.1007/s11356-024-34597-9. Li H, Han ZT, Deng Q, et al. 2023. Assessing the effectiveness of nanoscale zero-valent iron particles produced by green tea for Cr(VI)-contaminated groundwater remediation. Journal of Groundwater Science and Engineering, 11(1): 55−67. DOI: 10.26599/JGSE.2023.9280006. Li H, Han ZT, Ma CX, et al. 2015. Comparison of 1, 2, 3-trichloropropane reduction and oxidation by nanoscale zerovalent iron, zinc and activated persulfate. Journal of Groundwater Science and Engineering, 3(2): 156−163. DOI: 10.26599/ JGSE.2015.9280018. Li H, Qian Y, Han ZT, et al. 2023. Natural attenuation monitoring of 1, 2, 3- trichloropropane and benzene in the groundwater of organic pollution sites, Tianjin chemical plant and its environmental restoration suggestions. Geology in China, 50(5): 1446−1459. DOI: 10.12029/gc20220302001. Li H, Zhao YS, Han ZT, et al. 2015. Study on influence factors of remediation area of in-situ reaction zone injected with modified nanoiron. China Environmental Science, 35(4): 1135−1141. Liu XM. 2018. Enhance degradation of polycyclic aromatic hydrocarbons with persulfate activation by nano zerovalent iron. Dalian: Dalin University of Technology. Liu XY, Liu WT, Chi ZF. 2022. Reduced graphene oxide supported nanoscale zero-valent iron (nZVI/rGO) for in-situ remediation of Cr(VI)/nitrate-polluted aquifer. Journal of Water Process Engineering, 49: 103188−103203. DOI: 10.1016/j.jwpe.2022.103188. Merrill J P, Suchomel EJ, Varadhan S, et al. 2019. Development and validation of technologies for remediation of 1, 2, 3-trichloropropane in groundwater. Current Pollution Reports, 5(4): 1−10. DOI: 10.1007/s40726-019-00122-7. Ning Z, Zhang M, Zhang NN, et al. 2022. Metagenomic characterization of a novel enrichment culture responsible for dehalogenation of 1, 2, 3-trichloropropane to allyl chloride. Journal of Environmental Chemical Engineering, 10(6): 108907−108916. DOI: 10.1016/j.jece.2022.108907. Plessl K, Russ A, Vollprecht D. 2023. Application and development of zero-valent iron (ZVI) for groundwater and wastewater treatment. International Journal of Environmental Science and Technology, 20: 6913−6928. DOI: 10.1007/s13762-022-04536-7. Ren JG, Gao PC, Xu XJ, et al. 2021. Advances in remediation technology for chlorinated hydrocarbons contamination in groundwater. Research of Environmental Science, 34(7): 1641−1653. DOI: 10.13198/j.issn.1001-6929.2021.01.07. Ren LM. 2019. Study on removal of chromium (VI) polluted groundwater using xanthan gum stabilized graphene oxide-supported nanoscale zero-valent iron. Changchun: Jilin University. Sarathy V, Salter AJ, Nurmi JT, et al. 2010. Degradation of 1, 2, 3-trichloropropane (TCP): Hydrolysis, elimination, and reduction by iron and znic. Environmental Science and Technology, 44: 787−793. DOI: 10.1021/es902595j. Shang WX, Xu HY, Wang JW, et al. 2022. Microbial degradation of organochlorine pesticides. Chemistry and Bioengineering, 39(3): 12−18. DOI: 10.3969/j.issn.1672-5425.2022.03.003. Sun F, Zhu Y, Liu X, et al. 2023. Highly efficient removal of Se(IV) using reduced graphene oxide-supported nanoscale zero-valent iron (nZVI/rGO): Selenium removal mechanism. Environmental Science and Pollution Research, 30: 27560−27569. DOI: 10.1007/s11356-022-24226-8. Wang J, Liu X, Wang X, et al. 2022. Fe2+ activating persulfate selectively oxidized alcohols by biphasic/homogeneous reaction switch strategy. Chemical Papers, 76(11): 7229−7234. DOI: 10.1007/s11696-022-02366-y. Xing R. 2020. Performances and mechamisms of GO/nZVI nanocomposites for organic pollutants removal. Hangzhou: Zhejiang University. DOI: 10.27461/d.cnki.gzjdx.2020.001991. Xue W, Chen X, Liu H, et al. 2024. Activation of persulfate by biochar-supported sulfidized nanoscale zero-valent iron for degradation of ciprofloxacin in aqueous solution: Process optimization and degradation pathway. Environmental Science and Pollution Research, 31(7): 10950−10966. DOI: 10.1007/s11356-024-31931-z. Xue W, Li J, Chen X. et al. 2023. Recent advances in sulfidized nanoscale zero-valent iron materials for environmental remediation and challenges. Environmental Science and Pollution Research, 30(46): 101933−101962. DOI: 10.1007/s11356-023-29564-9. Yan SY, Chen YY, Xu HZ, et al. 2008. A novel advanced oxidation technology based on activated persulfate. Progress in Chemistry, 20(9): 1433−1437. Yang XY, Yao C, Liu H, et al. 2018. Study on methylene blue removal by graphene oxide supported magnetic-nano iron. Technology of Water Treatment, 44(10): 53−57. DOI: 10.16796/j.cnki.1000-3770.2018.10.011. Yu C, Lu X, Lu J, et al. 2023. Degradation of amaranth by persulfate activated with zero-valent iron: Influencing factors, response surface modeling. SN Applied Sciences, 5(1): 1−14. DOI: 10.1007/s42452-022-05097-7. Zhang M. 2019. Degradation of 1, 2, 3-trichloropropane by activated sodium persulfate using nano iron suspension synthesized by green tea. Shijiazhuang: Hebei GEO university. DOI: 10.27752/d.cnki.gsjzj.2019.000010. Zhang M, Ning Z, Guo CJ, et al. 2023. Using compound specific isotope analysis to decipher the 1, 2, 3-trichloropropane-to-Allyl chloride transformation by groundwater microbial communities. Environmental Pollution, 316(1): 120577−120583. DOI: 10.1016/j.envpol.2022.120577. -

 E-mail alert
E-mail alert Rss
Rss


 下载:
下载: