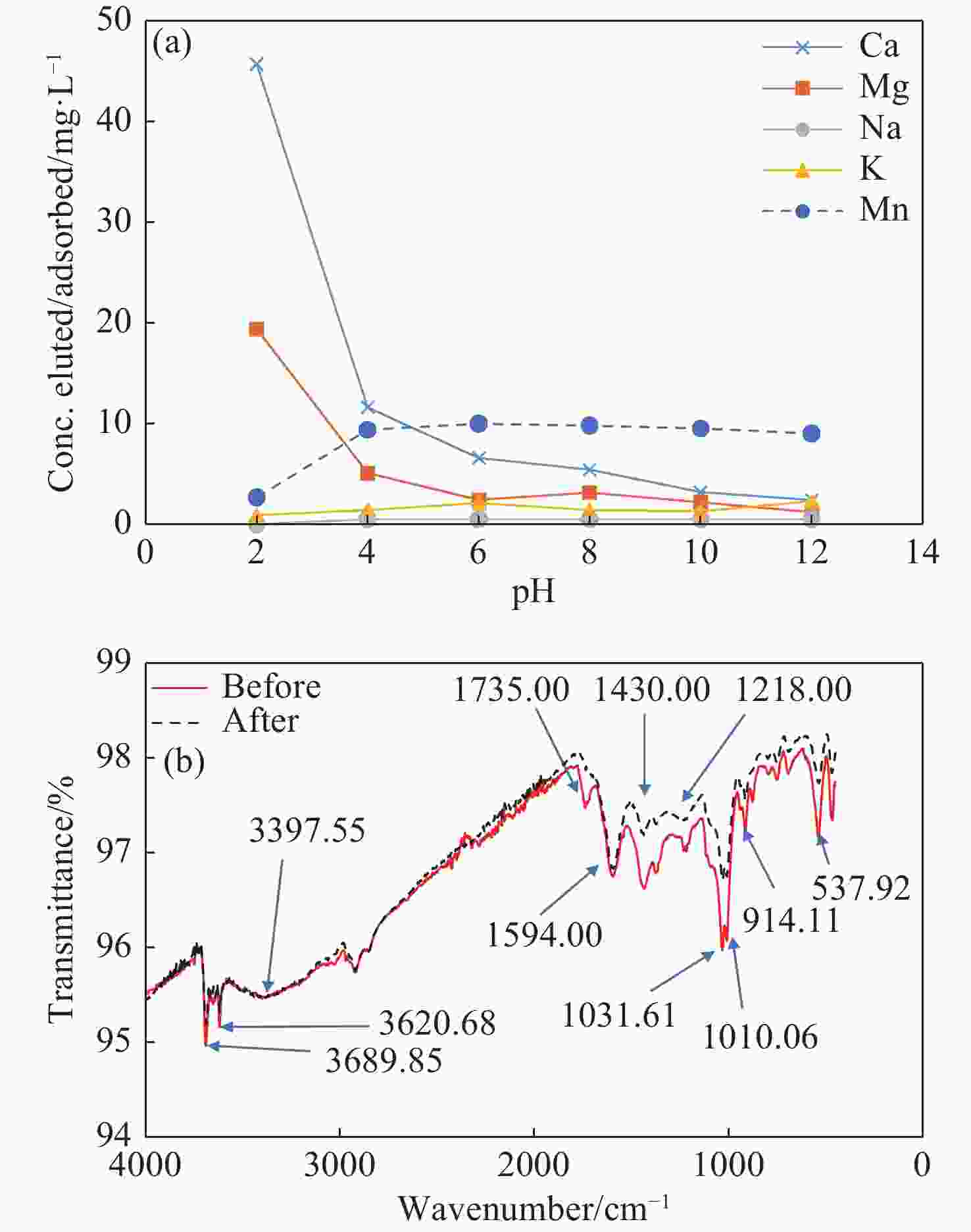
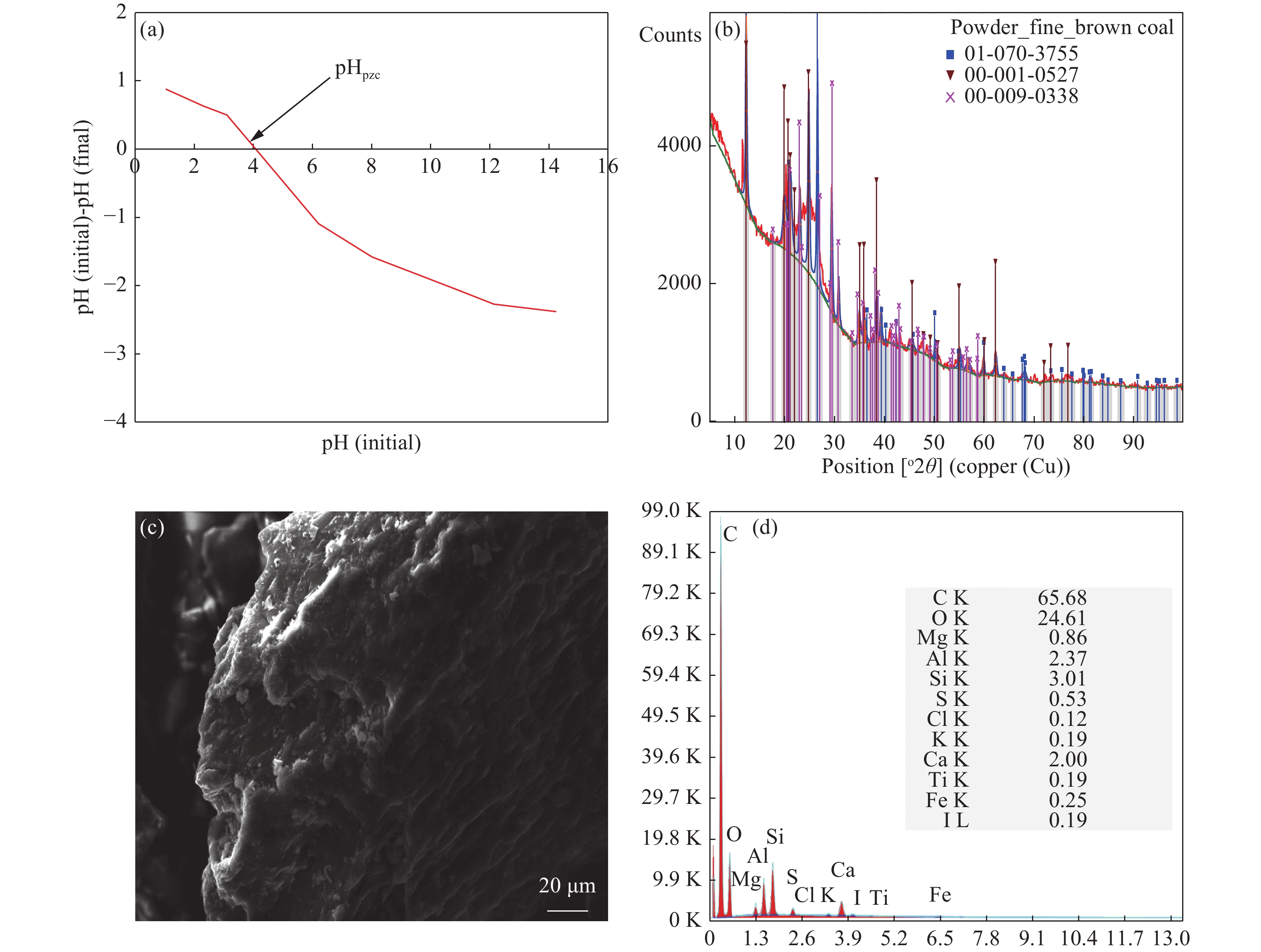
Natural brown coal as an adsorbent for manganese removal from groundwater: A mechanistic and operational evaluation
-
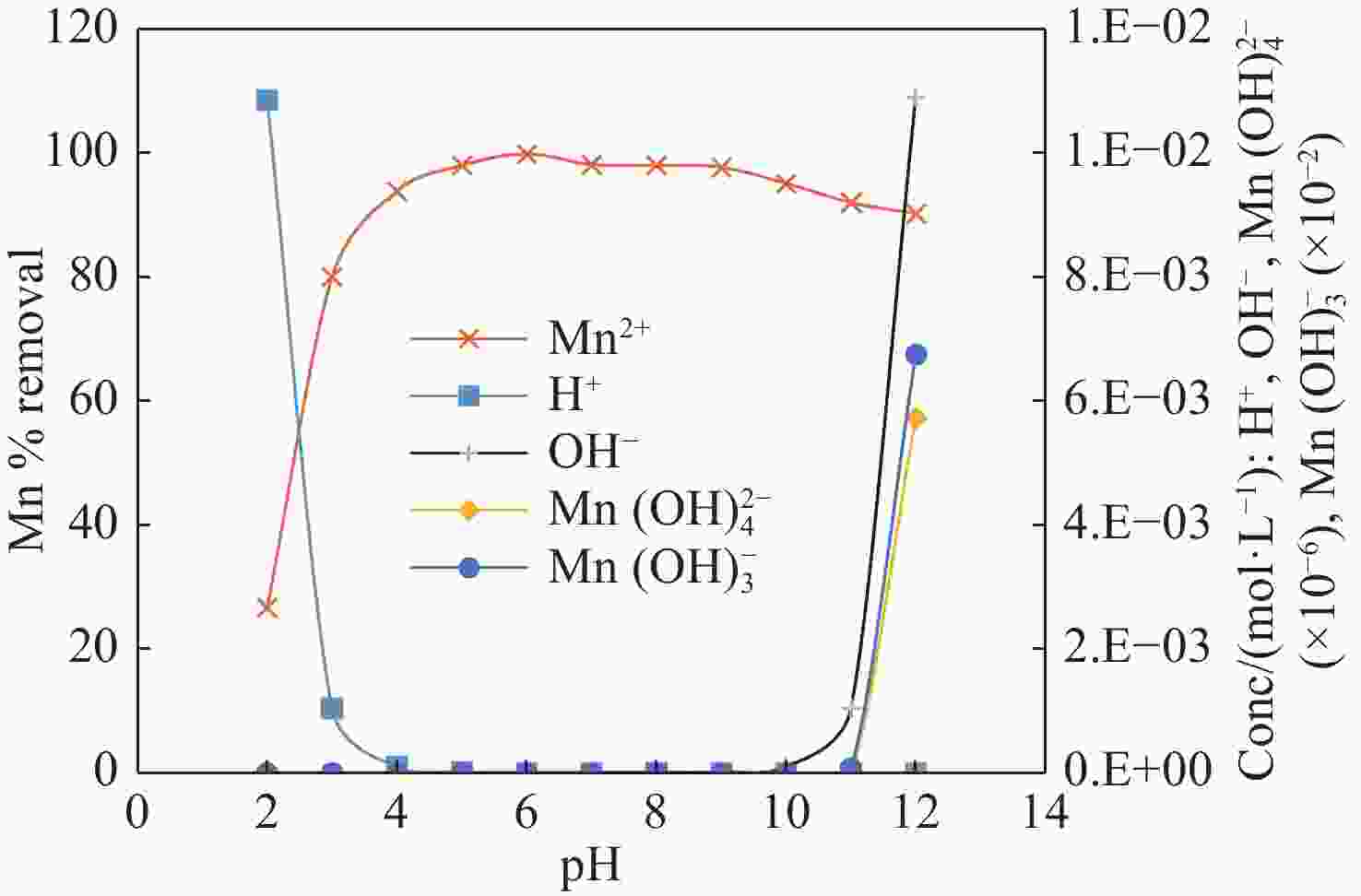
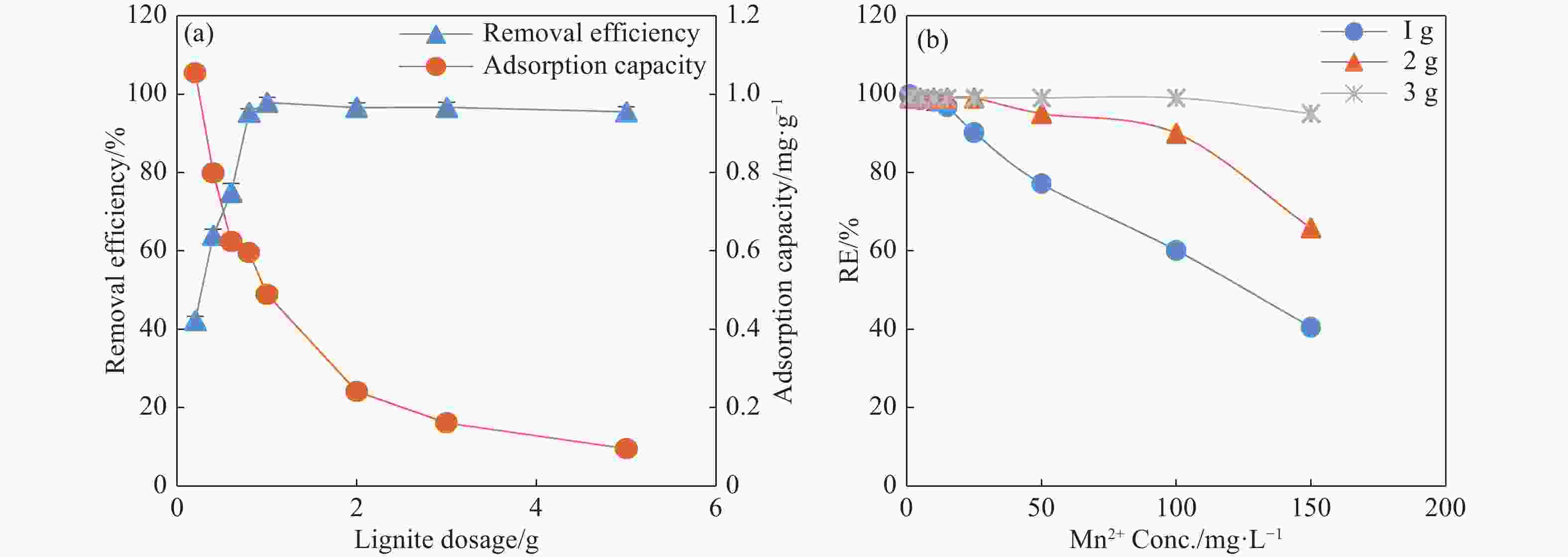
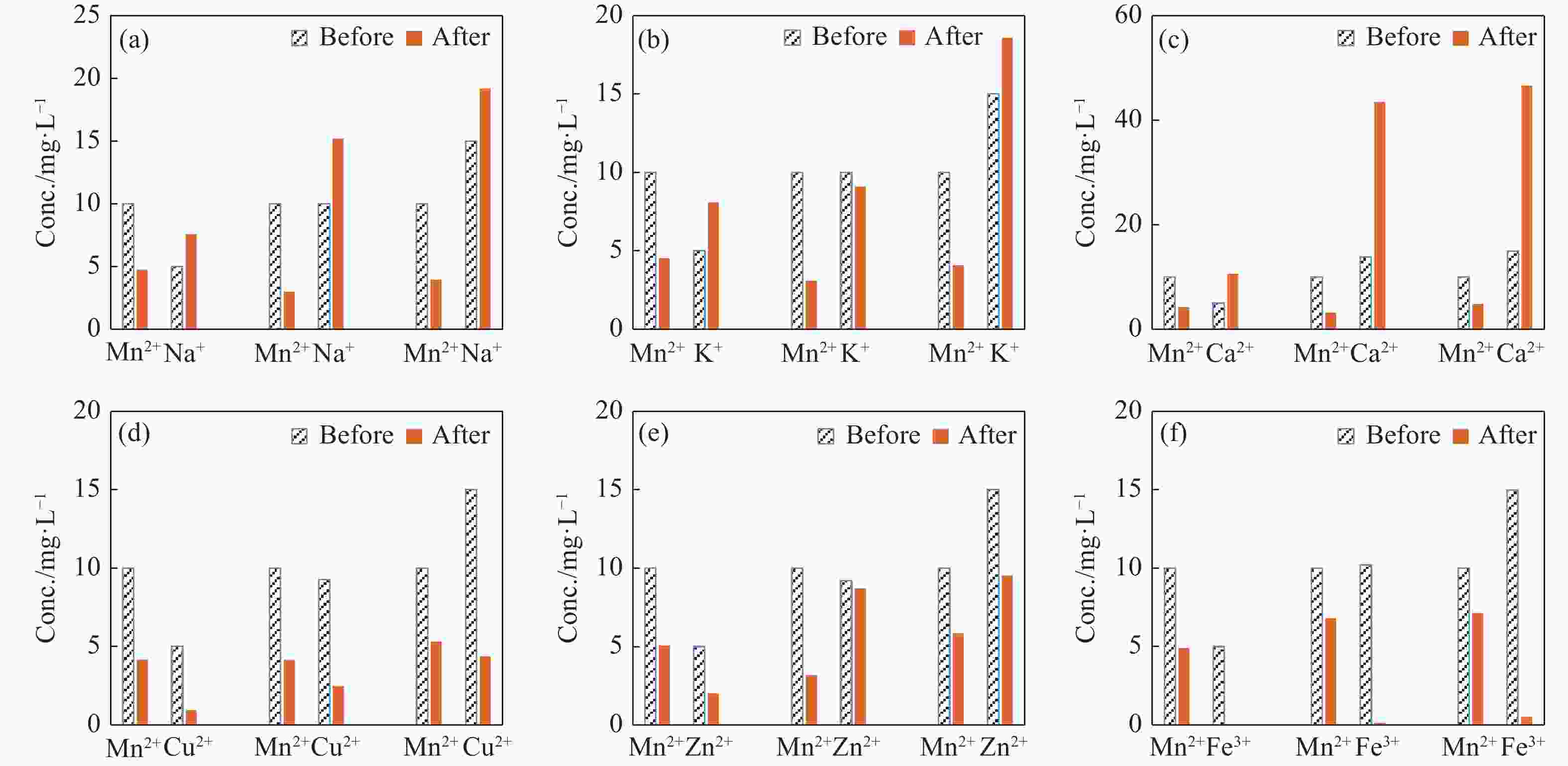
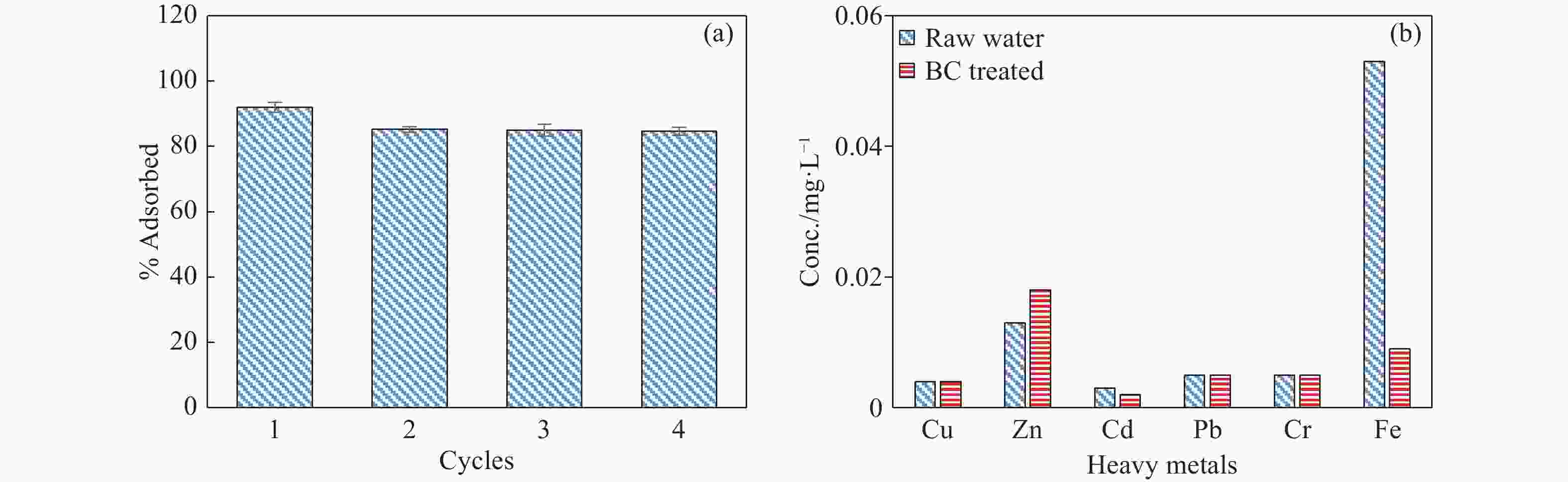
Abstract: This study investigates the potential of natural Brown Coal (BC) as a sustainable, cost-effective adsorbent for the removal of manganese (Mn2+) from contaminated groundwater. A series of batch adsorption experiments was conducted to assess the influence of key operational parameters—such as solution pH, initial Mn2+ concentration, BC dosage, temperature, and the presence of competing ions—on Mn2+ removal efficiency. The environmental compatibility and regeneration potential of BC were also evaluated to determine its practical viability for repeated use. To better understand the adsorption behaviour, equilibrium and kinetic data were analysed using established isotherm and kinetic models, while thermodynamic parameters were computed to assess the spontaneity and thermal characteristics of the adsorption process. Furthermore, geochemical modelling and comprehensive BC characterization—including surface morphology, mineralogical and elemental composition, and functional group analysis—were performed to elucidate Mn2+ speciation under varying environmental conditions and to uncover the underlying adsorption mechanisms. Results showed that Mn2+ removal efficiency increased with higher pH, temperature, and BC dosage, but declined at elevated initial Mn2+ concentrations due to active site saturation. The process was spontaneous and endothermic, with the Langmuir isotherm model (R2 = 0.994) and pseudo-second-order kinetic model (R2 = 0.996) providing the best fit to experimental data. Mechanistic analysis indicated that chemisorption, primarily through ion exchange and inner-sphere complexation, was the dominant mode of Mn2+ uptake. The presence of competing cations, especially Fe3+ and Cu2+, significantly hindered Mn2+ removal due to preferential binding. Importantly, BC exhibited strong reusability, maintaining over 80% removal efficiency across four adsorption–desorption cycles without evidence of secondary pollutants. These findings demonstrate the potential of natural BC as an efficient, reusable, and environmentally benign material for treating manganese-contaminated groundwater.
-
Key words:
- Sorption /
- Surface complexation /
- Ion exchange /
- Geochemical modelling /
- Heavy metals /
- Secondary pollution
-
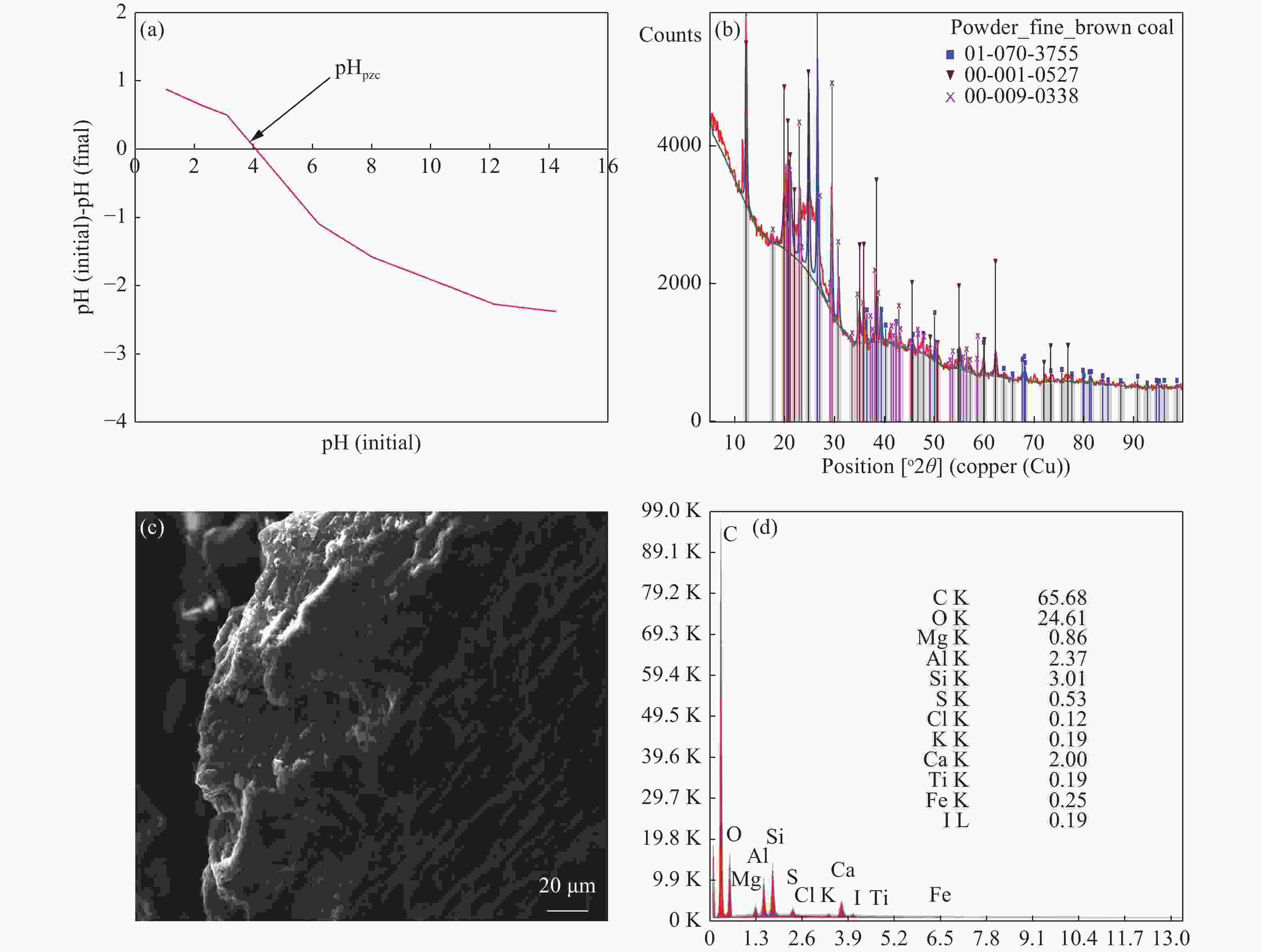
Table 1. Basic summary characteristics of the BC
Parameters Value pHpzc 4.02 Moisture (%) 5.30 Ash content (%) 7.90 Volatile matter (%) 30.10 Fixed carbon (%) 56.70 Pore volume (cm3/g) 0.017 Surface area (m2/g) 6.70 Elemental composition (%) Carbon 65.68 Oxygen 24.61 Ca 2.00 Si 3.01 Al 2.37 Table 2. Thermodynamic parameters for Mn2+ adsorption on BC
Temperature
K∆G○
kJ/mol∆H○
kJ/mol∆S○
kJ/mol-K298 −4.89 15.53 0.069 323 −6.73 348 −8.42 363 −9.12 373 −10.08 393 −11.55 Table 3. Comparison of Mn2+ adsorption capacity (Qmax) obtained in this study with those reported for other natural and low-cost adsorbents in previous studies
Adsorbent Qmax/mg/g Particle size pH Temperature/°C Reference Zeolite Y 0.015 0.75 µm 6.50 25 Kwakye-Awuah et al. 2019 Phoenix dactylifera L. seed 0.361 NA 7.0 NA Osundiya et al. 2024 Date palm biochar 0.44 0.15 mm 6 NA Fseha et al. 2022 Tea waste 0.158 2 mm NA NA Badrealam et al. 2019 BC 1.190 75 µm 6.00 25 This study Qmax: Maximum adsorption capacity; NA = not available Table 4. Parameters of the PFK, PSK and IPD models
PFK PSK IPD qe (mg/g) k1 (1/h) R2 qe (mg/g) k2 (mg/g h) R2 kp1 (mg/g min1/2) R2 kp2 (mg/g min1/2) R2 0.259 0.070 0.988 0.268 0.673 0.996 0.005 0.913 0.0008 0.722 Table 5. Physico-chemical properties of real groundwater before and after BC treatment
Parameter Unit Before BC After BC WHO (2017) Turbidity NTU 59.2 2.09 5 Colour (apparent) Hz 75.0 5.00 5 pH pH Units 6.21 6.57 6.5–8.5 Conductivity µS/cm 531 623 - TSS mg/l 49.0 1.00 - TDS mg/l 293 343 1,000 Sodium mg/l 16.0 56.0 200 Potassium mg/l 6.00 3.30 30 Calcium mg/l 24.0 28.9 200 Magnesium mg/l 21.7 20.7 150 Total Iron mg/l 18.0 0.401 0.3 Ammonium (NH4-N) mg/l 0.001 <0.001 0.00–1.5 Chloride mg/l 76.9 185 250 Sulphate (SO4) mg/l 18.7 16.10 250 Manganese mg/l 10.20 1.14 0.4 Nitrite (NO2-N) mg/l 0.036 <0.001 1 Nitrate (NO3-N) mg/l 0.111 0.247 10 Total Hardness (as CaCO3) mg/l 150 157 500 Bicarbonate (as CaCO3) mg/l 77.6 14.4 - -
Badrealam S, Darrell VC, Dollah Z, et al. 2019. Adsorption of manganese and zinc in synthetic wastewater by tea waste (TW) as a low-cost adsorbent. Journal of Physics: Conference Series, 1349(1): 012061. DOI: 10.1088/1742-6596/1349/1/012061. Biney E, Mintah BA, Ankomah E, et al. 2024. Sustainability assessment of groundwater in south-eastern parts of the western region of Ghana for water supply. Cleaner Water, 1: 100007. DOI: 10.1016/j.clwat.2024.100007. Caldwell RW, Rodriguez PC, Toque HA, et al. 2018. Arginase: A multifaceted enzyme important in health and disease. Physiological reviews, 98(2): 641−665. DOI: 10.1152/physrev.00037.2016. Çalışır A, Yavuz SÇ, Yavuz E, et al. 2024. Removal of manganese (Mn2+) from water samples using a biocomposite sorbent. Environmental Research, 257: 119353. DOI: 10.1016/j.envres.2024.119353. Chen JJ, Tao L, Liu BX, et al. 2024. Distribution characteristics and origin analysis of iron and manganese in groundwater in Beijing Plain Area. Hydrogeology & Engineering Geology, 51(6): 198−207. DOI: 10.16030/j.cnki.issn.1000-3665.202311051. Chen X, Hossain M, Duan C, et al. 2022. Isotherm models for adsorption of heavy metals from water-A review. Chemosphere, 307: 135545. DOI: 10.1016/j.chemosphere.2022.135545. Cui X, Fang S, Yao Y, et al. 2016. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Science of the Total Environment, 562: 517−525. DOI: 10.1016/j.scitotenv.2016.03.248. Debrah SK, Issahaku T, Obiri-Nyarko F, et al. 2024. Hydrogeochemical characterization and spatial analysis of groundwater quality in the Bono East Region, Ghana. International Journal of Energy and Water Resources, 1-18. DOI: 10.1007/s42108-024-00322-y Efome JE, Rana D, Matsuura T, et al. 2019. Effects of operating parameters and coexisting ions on the efficiency of heavy metal ions removal by nano-fibrous metal-organic framework membrane filtration process. Science of the Total Environment, 674: 355−362. DOI: 10.1016/j.scitotenv.2019.04.187. Fseha YH, Sizirici B, Yildiz I. 2022. Manganese and nitrate removal from groundwater using date palm biochar: Application for drinking water. Environmental Advances, 8: 100237. DOI: 10.1016/j.envadv.2022.100237. Galangashi MA, Kojidi SFM, Pendashteh A, et al. 2021. Removing iron, manganese and ammonium ions from water using greensand in fluidized bed process. Journal of Water Process Engineering, 39: 101714. DOI: 10.1016/j.jwpe.2020.101714. Hu J, Han B, Butterly CR, et al. 2024. Catalytic oxidation of lignite by Pt/TiO2 can enhance cadmium adsorption capacity. Journal of Hazardous Materials, 465: 133207. DOI: 10.1016/j.jhazmat.2023.133207. Jakariya M, Rahman MM, Mahzabin L, et al. 2024. Developing a safe water atlas for sustainable drinking water supply in Sonargaon Upazila, Bangladesh. Groundwater for Sustainable Development, 25: 101126. DOI: 10.1016/j.gsd.2024.101126. Jellali S, Azzaz AA, Jeguirim M, et al. 2021. Use of lignite as a low-cost material for cadmium and copper removal from aqueous solutions: Assessment of adsorption characteristics and exploration of involved mechanisms. Water, 13(2): 164. DOI: 10.3390/w13020164. Jiang L, Cheng Y, Huang T, et al. 2023. Removal of manganese from water by modified groundwater plant sludge: Mechanism and application as filter media. Journal of Water Process Engineering, 51: 103418. DOI: 10.1016/j.jwpe.2022.103418. Kim S, Gholamirad F, Yu M, et al. 2021. Enhanced adsorption performance for selected pharmaceutical compounds by sonicated Ti3C2TX MXene. Chemical Engineering Journal, 406: 126789. DOI: 10.1016/j.cej.2020.126789. Kobielska PA, Howarth AJ, Farha OK, et al. 2018. Metal–organic frameworks for heavy metal removal from water. Coordination Chemistry Reviews, 358: 92−107. DOI: 10.1016/j.ccr.2017.12.010. Kolya H, Kang CW. 2025. Recent advances in polymer nanocomposites for the adsorptive removal of toxic azo dyes from water. Discover Water, 5(1): 28. DOI: 10.1007/s43832-025-00217-x. Kurniasih M, Aprilita NH, Roto R, et al. 2025. Modification of coal fly ash for high-capacity adsorption of methylene blue. Case Studies in Chemical and Environmental Engineering, 11: 101101. DOI: 10.1016/j.cscee.2025.101101. Kwakye-Awuah B, Sefa-Ntiri B, Von-Kiti E, et al. 2019. Adsorptive removal of iron and manganese from groundwater samples in Ghana by zeolite Y synthesized from bauxite and kaolin. Water, 11(9): 1912. DOI: 10.3390/w11091912. Macena M, Pereira H, Cruz-Lopes L, et al. 2025. Competitive adsorption of metal ions by lignocellulosic materials: A review of applications, mechanisms and influencing factors. Separations, 12(3): 70. DOI: 10.3390/separations12030070. McKinley K, McLellan I, Gagné F, et al. 2019. The toxicity of potentially toxic elements (Cu, Fe, Mn, Zn and Ni) to the cnidarian Hydra attenuata at environmentally relevant concentrations. Science of the Total Environment, 665: 848−854. DOI: 10.1016/j.scitotenv.2019.02.193. Nadagouda MN, Varshney G, Varshney V, et al. 2024. Recent advances in technologies for phosphate removal and recovery: A review. ACS Environmental Au, 4(6): 271−291. DOI: 10.1021/acsenvironau.3c00069. Neculita CM, Rosa E. 2019. A review of the implications and challenges of manganese removal from mine drainage. Chemosphere, 214: 491−510. DOI: 10.1016/j.chemosphere.2018.09.106. Obiri-Nyarko F, Kwiatkowska-Malina J, Kumahor SK, et al. 2022. Evaluating low-cost permeable adsorptive barriers for the removal of benzene from groundwater: Laboratory experiments and numerical modelling. Journal of Contaminant Hydrology, 250: 104054. DOI: 10.1016/j.jconhyd.2022.104054. Osundiya M, Sobola A, Oyewole T, et al. 2024. Kinetics, isotherms and thermodynamics studies of Pb2+ and Mn2+ adsorption from model wastewater solution using raw Phoenix dactylifera L. seed. Journal of Research and Review in Science, 11: 30−46. DOI: 10.36108/jrrslasu/4202.11.0192. Panagopoulos A, Michailidis P. 2025. Membrane technologies for sustainable wastewater treatment: Advances, challenges, and applications in zero liquid discharge (zld) and minimal liquid discharge (mld) systems. Membranes, 15(2): 64. DOI: 10.3390/membranes15020064. Quansah JO, Obiri-Nyarko F, Karikari AY. 2024. Adsorptive removal of dissolved iron from groundwater by brown coal–A low-cost adsorbent. Journal of Contaminant Hydrology, 260: 104283. DOI: 10.1016/j.jconhyd.2023.104283. Rudi NN, Muhamad MS, Te Chuan L, et al. 2020. Evolution of adsorption process for manganese removal in water via agricultural waste adsorbents. Heliyon, 6(9): e05049. DOI: 10.1016/j.heliyon.2020.e05049 Sahmoune MN. 2019. Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Environmental Chemistry Letters, 17(2): 697−704. DOI: 10.1007/s10311-018-00819-z. Shi Z, Wu C, Wang F, et al. 2024. Adsorption performance and mechanism of Fe (II) adsorption in abandoned mine water of nonstick coal. Processes, 12(1): 188. DOI: 10.3390/pr12010188. Siri-Anusornsak W, Kolawole O, Soiklom S, et al. 2024. Innovative use of spirogyra sp biomass for the sustainable adsorption of aflatoxin B1 and ochratoxin A in aqueous solutions. Molecules, 29(21): 5038. DOI: 10.3390/molecules29215038. Sithole T. 2024. A review on regeneration of adsorbent and recovery of metals: Adsorbent disposal and regeneration mechanism. South African Journal of Chemical Engineering, 50(1): 39-50. DOI: 10.1016/j.sajce.2024.07.006 Sönmez D, Hocaoğlu Ç. 2023. Manganese intoxication presenting with depressive symptoms: A case report. Archives of Neuropsychiatry, 60(3): 288. DOI: 10.29399/npa.28305. Sun M, Gu S, Liu X, et al. 2023. Adsorption mechanism of ammonia nitrogen and phenol on lignite surface: Molecular dynamics simulations and quantum chemical calculations. Fuel, 337: 127157. DOI: 10.1016/j.fuel.2022.127157. Tabi RN, Gibrilla A, Boakye P, et al. 2024. Appraisal of groundwater quality and hydrochemistry in three regions of Ghana: Implications for drinking purposes. Groundwater for Sustainable Development, 26: 101193. DOI: 10.1016/j.gsd.2024.101193. Tu Y, Feng P, Ren Y, et al. 2019. Adsorption of ammonia nitrogen on lignite and its influence on coal water slurry preparation. Fuel, 238: 34−43. DOI: 10.1016/j.fuel.2018.10.085. Virolainen S, Wesselborg T, Kaukinen A, et al. 2021. Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometallurgy, 202: 105602. DOI: 10.1016/j.hydromet.2021.105602. Wang J, Guo X. 2020. Adsorption kinetic models: Physical meanings, applications, and solving methods. Journal of Hazardous materials, 390: 122156. DOI: 10.1016/j.jhazmat.2020.122156. WHO. 2017. World Health Organization. Guidelines for drinking-water quality: First addendum to the fourth edition. Xu P, Zeng GM, Huang DL, et al. 2017. Fabrication of reduced glutathione functionalized iron oxide nanoparticles for magnetic removal of Pb (II) from wastewater. Journal of the Taiwan Institute of Chemical Engineers, 71: 165−173. DOI: 10.1016/j.jtice.2016.11.031. Yao N, Li C, Yu J, et al. 2020. Insight into adsorption of combined antibiotic-heavy metal contaminants on graphene oxide in water. Separation and Purification Technology, 236: 116278. DOI: 10.1016/j.seppur.2019.116278. Zhan X, Zhang Q, Li M, et al. 2024. The shape of reactive nitrogen losses from intensive farmland in China. Science of the Total Environment, 915: 170014. DOI: 10.1016/j.scitotenv.2024.170014. Zhang H, Li Q, Zhao M, et al. 2025. Leaching law of heavy metals in coal gangue: A combination of experimental optimization and simulation. Journal of Hazardous Materials, 484: 136790. DOI: 10.1016/j.jhazmat.2024.136790. Zhang X, Zhou JW, Wang XJ, et al. 2025. Removal process of iron and manganese ions in acid mine water. Hydrogeology & Engineering Geology, 52(5): 1−10. DOI: 10.16030/j.cnki.issn.1000-3665.202505013. Zhang Z, Zhu M, Li J, et al. 2019. Effect of heat treatment on the combustion characteristics of a lignite. Journal of Energy Resources Technology, 141(7): 070705. DOI: 10.1115/1.4042823. Zhao Y, Naeth MA. 2024. Cd (II) and Zn (II) adsorption on lignite-derived humic substances and cattle manure biochar. CLEAN–Soil, Air, Water, 52(8): 2400226. DOI: 10.1002/clen.202400226. -
 E-mail alert
E-mail alert Rss
Rss


 下载:
下载: