Determination of total sulfur in geothermal water by inductively coupled plasma-atomic emission spectrometry
-
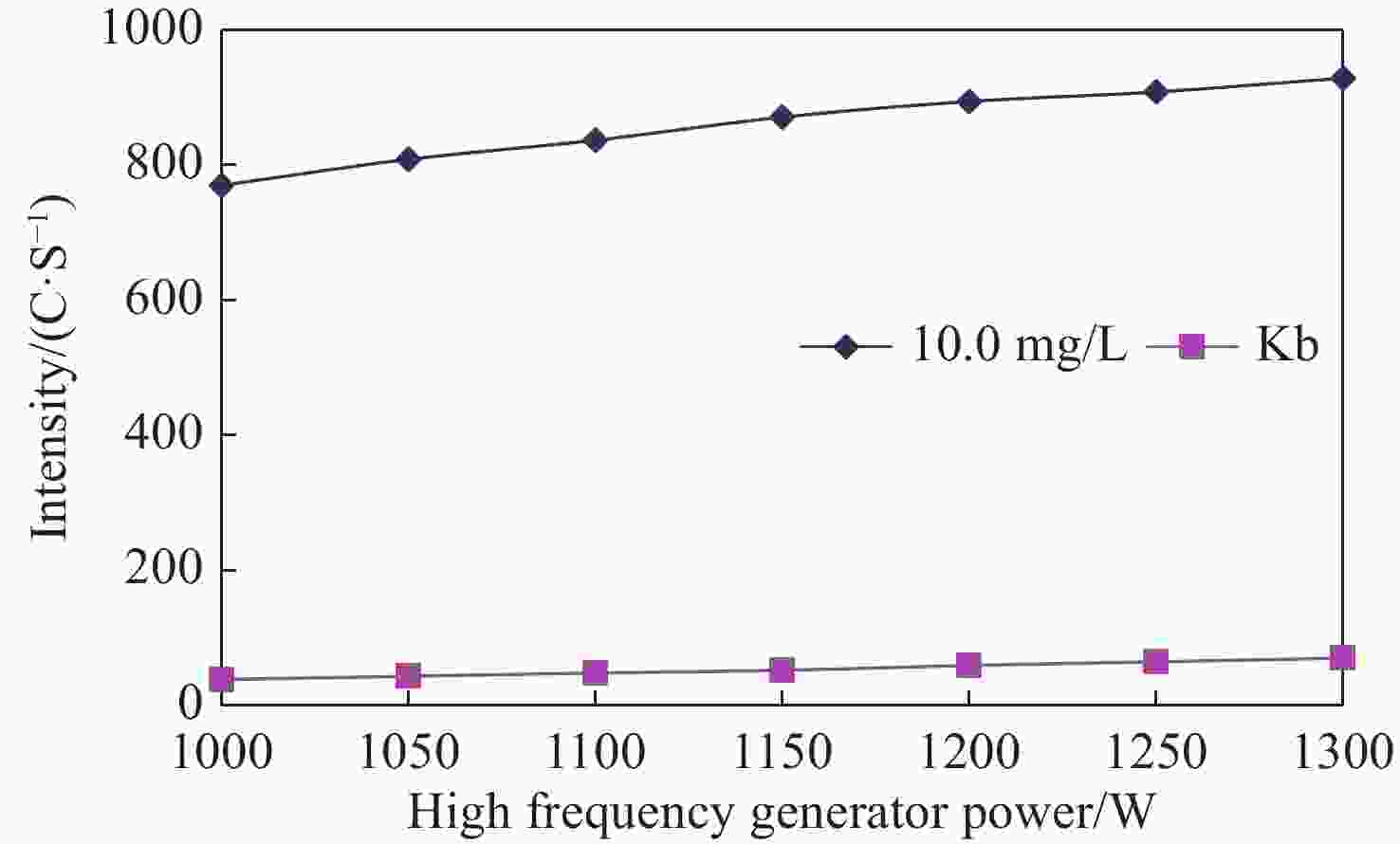
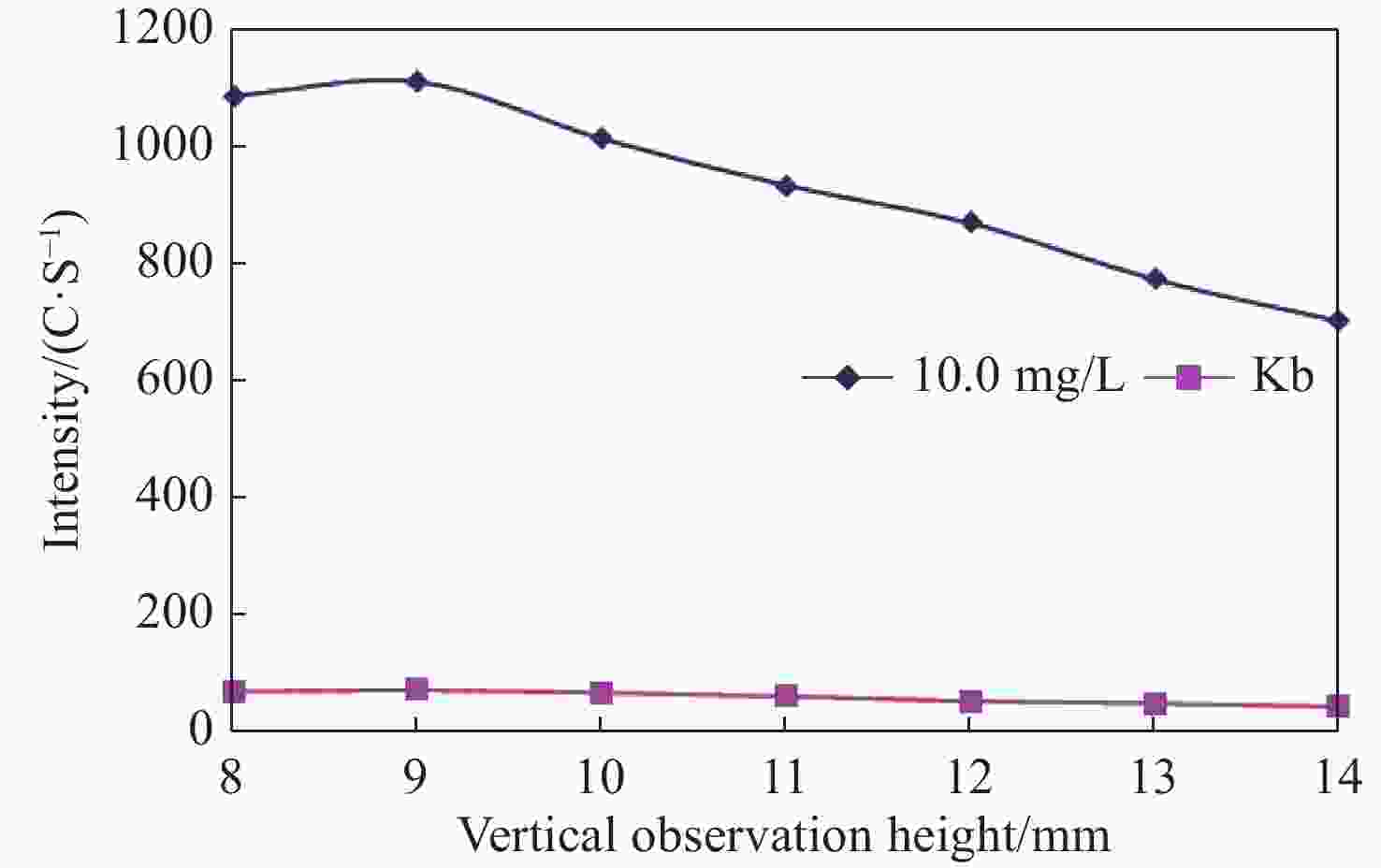
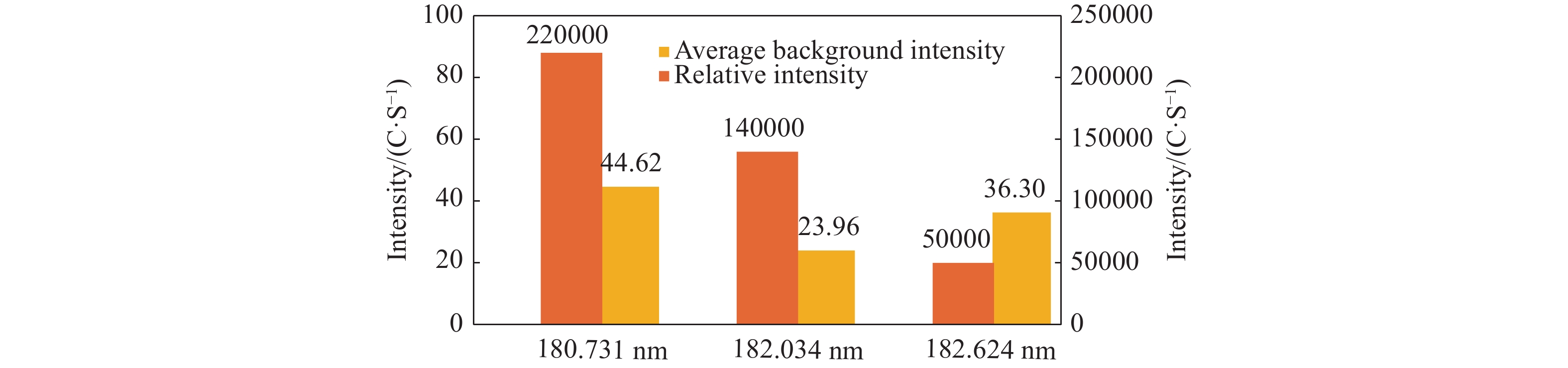
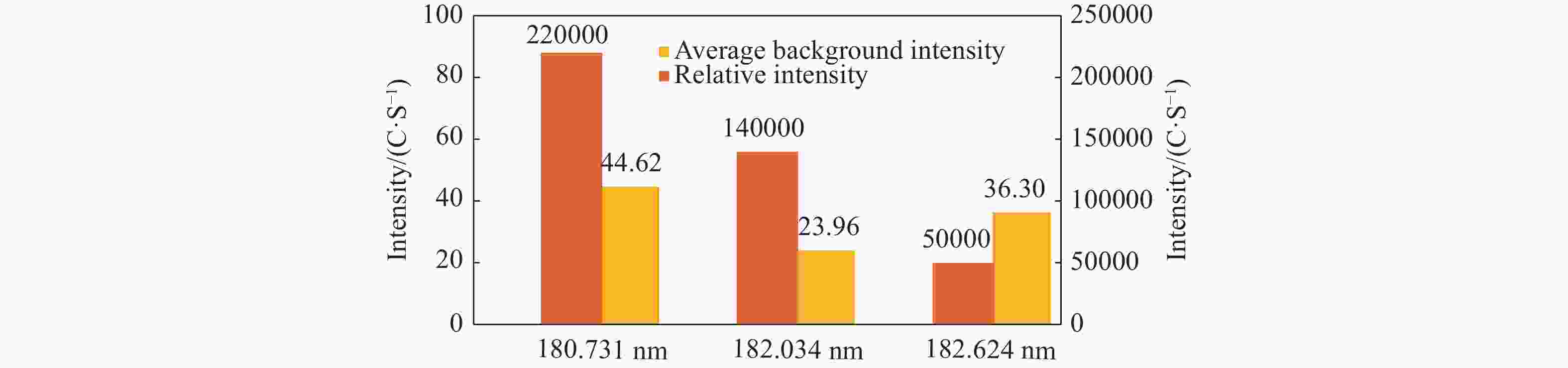
Abstract: Sulfur speciation and concentration in geothermal water are of great significance for the research and utilization of the water resources. In most situations, it is necessary to determine the total sulfur in geothermal water. In this study, the method was established for the determination of determining total sulfur content — the inductively coupled plasma-atomic emission spectrometry (ICP-AES), with the wavelength of 182.034 nm selected in spectral line of sulfur. It was identified that the optimal working conditions of the ICP-AES instrument were 1 200 W for high frequency generator power 9 mm for vertical observation height, 0.30 MPa atomizer pressure, and 50 r/min analytical pump speed. The matrix interference of the method was eliminated by the matrix matching method. Using this method, sulfur detection limit and minimum quantitative detection limit were 0.028 mg/L and 0.110 mg/L, respectively, whilst the linear range was 0.0–100.0 mg/L. The recovery rate of sample was between 90.67% and 108.7%, and the relative standard deviation (RSD) was between 0.36% and 2.14%. The method was used to analyze the actual samples and the results were basically consistent with the industry standard method. With high analysis efficiency, the method has low detection limit and minimum quantitative detection limit, wide linear range, good precision and accuracy, and provides an important detection method for the determination of total sulfur in geothermal water.
-
Table 1. Working conditions of instrument
Working conditions Parameter Working conditions Parameter High frequency generator power(W) 1 200 Auxiliary air flow(L/min) 0.50 Atomizer pressure(MPa) 0.30 Integration time(s) 15 Vertical observation height(mm) 9 Stable schedule(s) 5 Analytical pump speed(r/min) 50.0 Flushing pump speed(r/min) 50.0 Table 2. Selection of different atomizer pressure and analytical pump speed
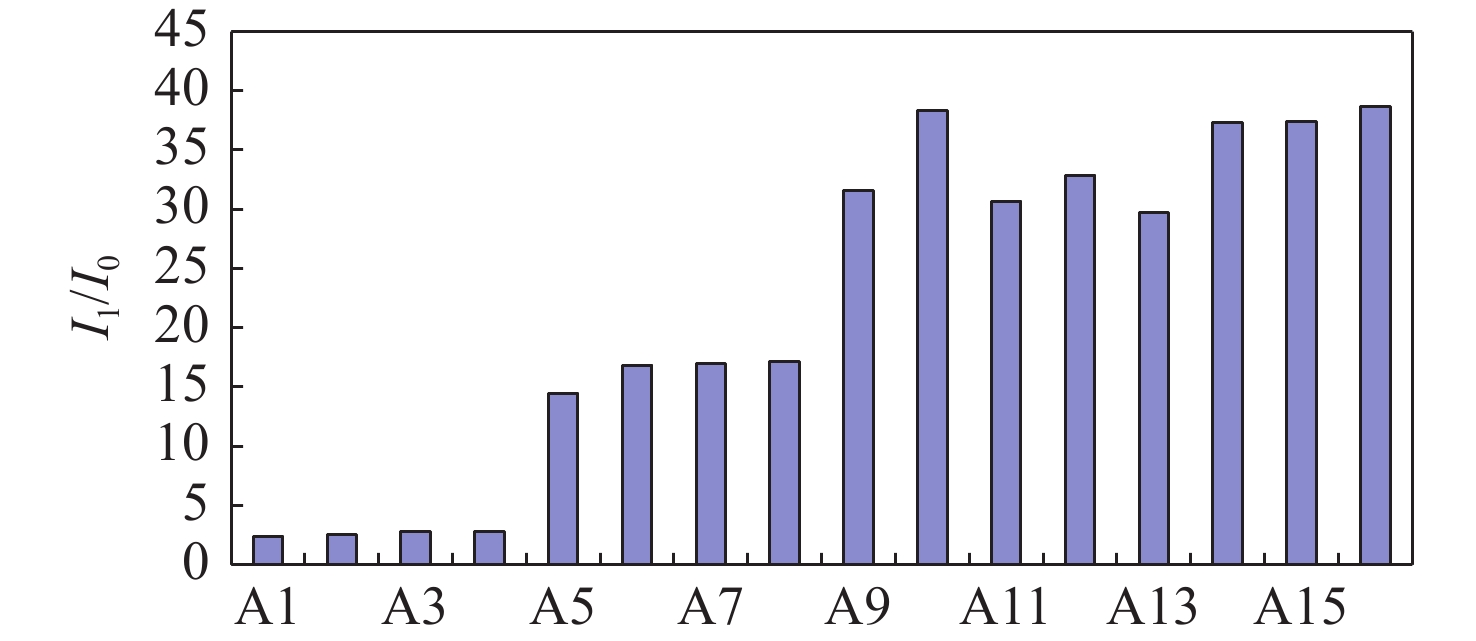
Atomizer pressure(MPa) Analytical pump speed(r/min) Atomizer pressure(MPa) Analytical pump speed(r/min) A1 0.10 25 A9 0.30 25 A2 0.10 50 A10 0.30 50 A3 0.10 75 A11 0.30 75 A4 0.10 100 A12 0.30 100 A5 0.20 25 A13 0.40 25 A6 0.20 50 A14 0.40 50 A7 0.20 75 A15 0.40 75 A8 0.20 100 A16 0.40 100 Table 3. Determination results of total sulfur in geothermal water samples by matrix matching method and standard addition method (n=6)
Linear regression equation Correlation coefficient Linear range (mg/L) Sample 1# (mg/L) Sample 2# (mg/L) Sample 3# (mg/L) Matrix matching method Y=77.378x+14.592 0.9999 0.0–100.0 3.056 8.410 29.802 Standard addition method Y=70.220x+131.58 0.9999 0.0–100.0 2.997 8.277 30.441 Relative deviation(%) -- -- -- 1.95 1.59 2.12 Table 4. Method detection limit, lower limit of determination and linear range compared with DZ/T 0064.51-2021
ICP Spectroscopy(as total sulphur)(this study) Ion chromatography(as SO42−)(DZ/T 0064.51-2021) Detection limit (mg/L) 0.028 0.10 Minimum quantitative detection limit (mg/L) 0.110 0.30 Linear range (mg/L) 0.0–100.0 0.30–50.00 Table 5. Method precision and accuracy test (n=6)
Sample number Measured value(mg/L) Average value(mg/L) RSD
(%)Added value(mg/L) Recovery rate(%) RSD
(%)B1# 8.89, 9.05, 9.15, 9.10, 8.78, 9.12 9.02 1.63 10.0 91.60–101.1 1.89 B2# 13.70, 13.74, 13.29, 13.54, 13.57, 13.11 13.49 1.82 15.0 95.87–108.7 2.14 B3# 40.75, 40.87, 41.04, 41.01, 40.97, 40.68 40.89 0.36 10.0 91.20–103.9 0.88 B4# 21.66, 20.96, 21.14, 21.65, 21.78, 21.10 21.38 1.65 5.0 91.40–108.0 1.13 B5# 240.5, 237.2, 239.7, 236.2, 244.1, 241.9 239.9 1.22 45.0 90.67–107.4 1.15 Table 6. Determination of total sulfur in geothermal water samples from different regions (n=6)
Sample number ICP Spectroscopy DZ/T 0064.51-2021 Relative deviation(%) Measured value(mg/L) RSD(%) Measured value(mg/L) RSD(%) C1# 22.96 1.20 23.40 0.69 1.89 C2# 54.78 1.04 53.49 1.48 2.39 C3# 89.58 2.84 90.35 0.63 0.85 C4# 1.98 1.41 2.05 3.75 3.73 C5# 2383 0.31 2401 2.53 0.75 C6# 71.68 1.07 71.47 0.65 0.30 -
Ahmadi M, Aguirre MÁ, Madrakian T, et al. 2017. Total sulfur determination in liquid fuels by ICP-OES after oxidation-extraction desulfurization using magnetic graphene oxide. Fuel, 210: 507−513. doi: 10.1016/j.fuel.2017.08.104 Banks D, Boyce AJ, Westaway R, et al. 2021. Sulphur isotopes in deep groundwater reservoirs: Evidence from post-stimulation flowback at the Pohang geothermal facility, Korea. Geothermics, 91: 102003. doi: 10.1016/j.geothermics.2020.102003 Guo L, Zhao HY, Wen HY, et al. 2012. Simulataneous determination of Li, Na, K, Ca, Mg, B, S, Cl in brine by inductively coupled plasma-atomic emission spectrometry. Rock and Mineral Analysis, 31(5): 824−828. (in Chinese) doi: 10.3969/j.issn.0254-5357.2012.05.012 Hammerli J, Greber ND, Martin L, et al. 2021. Tracing sulfur sources in the crust via SIMS measurements of sulfur isotopes in apatite. Chemical Geology, 579: 120242. doi: 10.1016/j.chemgeo.2021.120242 Hu X, Shi L, Zhang WH. 2017. Determination of sulfur in high-sulfur bauxite by alkali fusion-inductively coupled plasma optical emission spectrometry. Rock and Mineral Analysis, 36(2): 124−129. (in Chinese) doi: 10.15898/j.cnki.11-2131/td.2017.02.005 Hu JZ, Wang L, Liu J, et al. 2018. Determination of water soluble sulfate in soil by inductively coupled plasma atomic emission spectrometry. Metallurgical Analysis, 38(11): 12−17. (in Chinese) doi: 10.13228/j.boyuan.issn1000-7571.010473 Kapitány S, Nagy D, Posta J, et al. 2020. Determination of atmospheric sulphur dioxide and sulphuric acid traces by indirect flame atomic absorption method. Microchemical Journal, 157: 104853. doi: 10.1016/j.microc.2020.104853 Li QC, Zhao QL, AN MG, et al. 2017. Determination of sulfide in geothermal water by inductively coupled plasma-optical emission spectrometry. Rock and Mineral Analysis, 36(3): 239−245. (in Chinese) doi: 10.15898/j.cnki.11-2131/td.201612120181 Liu M, Guo Q, Zhang C, et al. 2017. Sulfur isotope geochemistry indicating the source of dissolved sulfate in gonghe geothermal waters, northwestern China. Procedia Earth and Planetary Science, 17: 157−160. doi: 10.1016/j.proeps.2016.12.039 Marrocos VCP, Gonçalves RA, Lepri FG, et al. 2020. Chemical modification for sulfur determination in human hair by high-resolution continuum source graphite furnace molecular absorption spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy, 174: 106008. doi: 10.1016/j.sab.2020.106008 Mitchell SC. 2021. Nutrition and sulfur. Advances in Food and Nutrition Research, 96: 123−174. doi: 10.1016/bs.afnr.2021.02.014 Ozbek N, Baysal A. 2015. A new approach for the determination of sulphur in food samples by high-resolution continuum source flame atomic absorption spectrometer. Food Chemistry, 168: 460−463. doi: 10.1016/j.foodchem.2014.07.093 Ozbek N, Akman S. 2016. Determination of total sulfur concentrations in different types of vinegars using high resolution flame molecular absorption spectrometry. Food Chemistry, 213: 529−533. doi: 10.1016/j.foodchem.2016.07.007 Robin JG, Stefánsson A, Ono S, et al. 2020. H2S sequestration traced by sulfur isotopes at Hellisheiði geothermal system, Iceland. Geothermics, 83: 101730. doi: 10.1016/j.geothermics.2019.101730 Schurr SL, Genske F, Strauss H, et al. 2020. A comparison of sulfur isotope measurements of geologic materials by inductively coupled plasma and gas source mass spectrometry. Chemical Geology, 558: 119869. doi: 10.1016/j.chemgeo.2020.119869 Wang LJ, Shi H. 2014. Direct determination of sulfate in natural mineral water by ICP-AES. Chinese Journal of Inorganic Analytical Chemistry, 4(4): 16−17. (in Chinese) doi: 10.3969/j.issn.2095-1035.2014.04.005 Wang XW, Liu JF, Guan H, et al. 2016. Determination of total sulfur dioxide in Chinese herbal medicines via triple quadrupole inductively coupled plasma mass spectrometry. Spectroscopy and Spectral Analysis, 36(2): 527−531. (in Chinese) doi: 10.3964/j.issn.1000-0593(2016)02-0527-05 Wang XF, Wang JJ. 2020. Determination of sulfur in soil by microwave digestion-inductively coupled plasma atomic emission spectrometry. Chemical Analysis and Meterage, 29(3): 47−50. (in Chinese) doi: 10.3969/j.issn.1008-6145.2020.03.011 Wang XJ, Liang WY, Xia MW, et al. 2021. Determination of total fluorine, total chlorine, total bromine and total sulfur in liquid hazardous waste. Chemical Reagents, 43(7): 936−940. (in Chinese) doi: 10.13822/j.cnki.hxsj.2021008006 Xu GR, Qu JG, Chang Y, et al. 2016. Accurate determination of sulfur content in sediments by double focusing inductively coupled plasma mass spectrometry combined with microwave digestion and studies on related matrix effect. Chinese Journal of Analytical Chemistry, 44(2): 273−280. (in Chinese) doi: 10.11895/j.issn.0253-3820.150735 Zhang WL, Long P, Wu J, et al. 2017. Determination of sulfur in solid and solution of phosphate ore pulp flue gas desulfurization agent with ICP-AES. Spectroscopy and Spectral Analysis, 37(5): 1535−1539. (in Chinese) doi: 10.3964/j.issn.1000-0593(2017)05-1535-05 -
 E-mail alert
E-mail alert Rss
Rss


 下载:
下载: